Synthetic di-sulfated iduronic acid attenuates asthmatic response by blocking T-cell recruitment to inflammatory sites
- PMID: 24835176
- PMCID: PMC4050563
- DOI: 10.1073/pnas.1319870111
Synthetic di-sulfated iduronic acid attenuates asthmatic response by blocking T-cell recruitment to inflammatory sites
Abstract
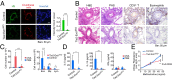
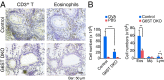
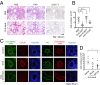
Identification of carbohydrate sequences that determine affinity to specific chemokines is a critical step for strategies to interfere with chemokine-mediated leukocyte trafficking. Here, we first characterized the development of allergic asthma in Tie2-dependent and inducible Ext1-knockout (Tie2-Ext1(iKO)) mice. We showed that heparan sulfate is essential for leukocyte recruitment in the peribronchial region and bronchoalveolar lavage fluid (BALF), and is crucial for induction of airway hyperresponsiveness. Our glycan microarray showed a unique affinity profile of chemokine CCL20 to substructures of heparin and heparin-like oligo/di/monosaccharides. Among them, we identified a synthetic and not naturally occurring monosaccharide, 2,4-O-di-sulfated iduronic acid (Di-S-IdoA), as a potential inhibitor for CCL20-heparan sulfate interaction. Mice injected with Di-S-IdoA via tail vain or nasal inhalation showed attenuated leukocyte recruitment into inflammatory sites and BALF. These results demonstrate a critical role of chemokine-heparan sulfate interaction in the asthma development and Di-S-IdoA as a potential drug for asthma treatment.
Keywords: lymphocyte recruitment; sulfated monosaccharide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TNFα-blockade stabilizes local airway hyperresponsiveness during TLR-induced exacerbations in murine model of asthma.Respir Res. 2015 Oct 22;16:129. doi: 10.1186/s12931-015-0292-5. Respir Res. 2015. PMID: 26494305 Free PMC article.
-
Elevated CCR6+ CD4+ T lymphocytes in tissue compared with blood and induction of CCL20 during the asthmatic late response.Clin Exp Immunol. 2008 Jun;152(3):440-7. doi: 10.1111/j.1365-2249.2008.03657.x. Epub 2008 Apr 16. Clin Exp Immunol. 2008. PMID: 18422729 Free PMC article.
-
DGKα DNA vaccine relieves airway allergic inflammation in asthma model possibly via induction of T cell anergy.Int J Clin Exp Pathol. 2013 Oct 15;6(11):2404-11. eCollection 2013. Int J Clin Exp Pathol. 2013. PMID: 24228102 Free PMC article.
-
Synthetic Approaches to L-Iduronic Acid and L-Idose: Key Building Blocks for the Preparation of Glycosaminoglycan Oligosaccharides.Adv Carbohydr Chem Biochem. 2015;72:21-61. doi: 10.1016/bs.accb.2015.07.001. Epub 2015 Oct 24. Adv Carbohydr Chem Biochem. 2015. PMID: 26613814 Review.
-
Glucuronyl C5-epimerase an enzyme converting glucuronic acid to iduronic acid in heparan sulfate/heparin biosynthesis.Prog Mol Biol Transl Sci. 2010;93:59-78. doi: 10.1016/S1877-1173(10)93004-4. Prog Mol Biol Transl Sci. 2010. PMID: 20807641 Review.
Cited by
-
Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System.Pharmaceuticals (Basel). 2017 Aug 9;10(3):70. doi: 10.3390/ph10030070. Pharmaceuticals (Basel). 2017. PMID: 28792472 Free PMC article. Review.
-
Hereditary multiple exostoses of the ribs as an uncommon cause of pneumothorax: A case report.Medicine (Baltimore). 2018 Aug;97(35):e11894. doi: 10.1097/MD.0000000000011894. Medicine (Baltimore). 2018. PMID: 30170381 Free PMC article.
-
Crude Turmeric Extract Improves the Suppressive Effects of Lactobacillus rhamnosus GG on Allergic Inflammation in a Murine Model of House Dust Mite-Induced Asthma.Front Immunol. 2020 Jun 4;11:1092. doi: 10.3389/fimmu.2020.01092. eCollection 2020. Front Immunol. 2020. PMID: 32582180 Free PMC article.
-
Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation.Int J Mol Sci. 2019 Apr 22;20(8):1963. doi: 10.3390/ijms20081963. Int J Mol Sci. 2019. PMID: 31013618 Free PMC article. Review.
-
Glycosaminoglycan microarrays for studying glycosaminoglycan-protein systems.Carbohydr Polym. 2024 Jul 1;335:122106. doi: 10.1016/j.carbpol.2024.122106. Epub 2024 Mar 29. Carbohydr Polym. 2024. PMID: 38616080 Review.
References
-
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–362. - PubMed
-
- Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: The ins and outs. Annu Rev Immunol. 2008;26:205–232. - PubMed
-
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100(11) Suppl:S97–S103. - PubMed
-
- Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8(5):362–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

