Kaposi's sarcoma-associated herpesvirus-encoded LANA can induce chromosomal instability through targeted degradation of the mitotic checkpoint kinase Bub1
- PMID: 24741095
- PMCID: PMC4054434
- DOI: 10.1128/JVI.00554-14
Kaposi's sarcoma-associated herpesvirus-encoded LANA can induce chromosomal instability through targeted degradation of the mitotic checkpoint kinase Bub1
Abstract
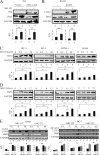
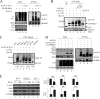
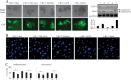
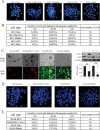
Kaposi's sarcoma-associated herpesvirus (KSHV) has a significant contributory role in the development of three major human neoplastic or lymphoproliferative diseases: Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD). These diseases are associated with chromosomal instability, a hallmark of human cancer. The latency-associated nuclear antigen (LANA) encoded by KSHV plays a key role in regulating a number of cellular pathways critical for oncogenesis. KSHV LANA alone can induce the development of B-cell hyperplasia and lymphoma in mice expressing LANA. LANA also induces chromosomal instability, thus promoting oncogenesis. However, the precise mechanism underlying LANA-mediated chromosomal instability remains uncharted. Here we report that LANA promoted the induction of chromosomal instability and the formation of micronuclei and multinucleation through its interaction with one of the critical spindle checkpoint proteins, Bub1, and the resulting degradation of Bub1. This interaction occurs through the Knl and kinase domains of Bub1, identified as important for stability and degradation. These results suggest that LANA can dysregulate Bub1 activity, which leads to aberrant chromosome replication and aneuploidy, thus contributing to KSHV-mediated oncogenesis.
Importance: This work represents the first set of results identifying a novel mechanism by which LANA, a latency-associated antigen encoded by KSHV, can induce the degradation of Bub1, a spindle checkpoint protein that is important for spindle checkpoint signaling and chromosome segregation. The downregulation of Bub1 mediated by LANA resulted in chromosomal instability, a hallmark of cancer. We further investigated the specific domains of Bub1 that are required for the interaction between LANA and Bub1. The results demonstrated that the Knl and kinase domains of Bub1 are required for the interaction between LANA and Bub1. In addition, we also investigated the mechanism by which LANA promoted Bub1 degradation. Our results showed that LANA interacted physically with the anaphase-promoting complex (APC/C), thus promoting the degradation of Bub1 in a ubiquitin-dependent process.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Bub1 in Complex with LANA Recruits PCNA To Regulate Kaposi's Sarcoma-Associated Herpesvirus Latent Replication and DNA Translesion Synthesis.J Virol. 2015 Oct;89(20):10206-18. doi: 10.1128/JVI.01524-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223641 Free PMC article.
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded LANA contributes to viral latent replication by activating phosphorylation of survivin.J Virol. 2014 Apr;88(8):4204-17. doi: 10.1128/JVI.03855-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478433 Free PMC article.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
-
Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies.Transl Res. 2013 Aug;162(2):77-92. doi: 10.1016/j.trsl.2013.03.004. Epub 2013 Apr 6. Transl Res. 2013. PMID: 23567332 Free PMC article. Review.
Cited by
-
Bub1 in Complex with LANA Recruits PCNA To Regulate Kaposi's Sarcoma-Associated Herpesvirus Latent Replication and DNA Translesion Synthesis.J Virol. 2015 Oct;89(20):10206-18. doi: 10.1128/JVI.01524-15. Epub 2015 Jul 29. J Virol. 2015. PMID: 26223641 Free PMC article.
-
Kaposi's Sarcoma Herpesvirus Genome Persistence.Front Microbiol. 2016 Aug 12;7:1149. doi: 10.3389/fmicb.2016.01149. eCollection 2016. Front Microbiol. 2016. PMID: 27570517 Free PMC article. Review.
-
The Modulation of Apoptotic Pathways by Gammaherpesviruses.Front Microbiol. 2016 Apr 27;7:585. doi: 10.3389/fmicb.2016.00585. eCollection 2016. Front Microbiol. 2016. PMID: 27199919 Free PMC article. Review.
-
Major Histocompatibility Complex Class II HLA-DRα Is Downregulated by Kaposi's Sarcoma-Associated Herpesvirus-Encoded Lytic Transactivator RTA and MARCH8.J Virol. 2016 Aug 26;90(18):8047-58. doi: 10.1128/JVI.01079-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27356905 Free PMC article.
-
Cellular Transformation by Human Cytomegalovirus.Cancers (Basel). 2024 May 22;16(11):1970. doi: 10.3390/cancers16111970. Cancers (Basel). 2024. PMID: 38893091 Free PMC article.
References
-
- Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648–2654 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

