An important role for mitochondrial antiviral signaling protein in the Kaposi's sarcoma-associated herpesvirus life cycle
- PMID: 24623417
- PMCID: PMC4019080
- DOI: 10.1128/JVI.03226-13
An important role for mitochondrial antiviral signaling protein in the Kaposi's sarcoma-associated herpesvirus life cycle
Abstract
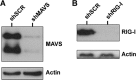
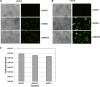
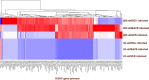
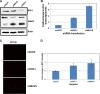
Kaposi's sarcoma-associated herpesvirus (KSHV) has been shown to be recognized by two families of pattern recognition receptors (PRRs), Toll-like receptors (TLRs) and NOD-like receptors (NLRs). Here we show that MAVS and RIG-I (retinoic acid-inducible gene 1), an RLR family member, also have a role in suppressing KSHV replication and production. In the context of primary infection, we show that in cells with depleted levels of MAVS or RIG-I, KSHV transcription is increased, while beta interferon (IFN-β) induction is attenuated. We also observed that MAVS and RIG-I are critical during the process of reactivation. Depletion of MAVS and RIG-I prior to reactivation led to increased viral load and production of infectious virus. Finally, MAVS depletion in latent KSHV-infected B cells leads to increased viral gene transcription. Overall, this study suggests a role for MAVS and RIG-I signaling during different stages of the KSHV life cycle.
Importance: We show that RIG-I and its adaptor protein, MAVS, can sense KSHV infection and that these proteins can suppress KSHV replication following primary infection and/or viral reactivation.
Figures

Similar articles
-
RIG-I Detects Kaposi's Sarcoma-Associated Herpesvirus Transcripts in a RNA Polymerase III-Independent Manner.mBio. 2018 Jul 3;9(4):e00823-18. doi: 10.1128/mBio.00823-18. mBio. 2018. PMID: 29970461 Free PMC article.
-
DExD/H Box Helicases DDX24 and DDX49 Inhibit Reactivation of Kaposi's Sarcoma Associated Herpesvirus by Interacting with Viral mRNAs.Viruses. 2022 Sep 20;14(10):2083. doi: 10.3390/v14102083. Viruses. 2022. PMID: 36298642 Free PMC article.
-
ADAR1 Facilitates KSHV Lytic Reactivation by Modulating the RLR-Dependent Signaling Pathway.Cell Rep. 2020 Apr 28;31(4):107564. doi: 10.1016/j.celrep.2020.107564. Cell Rep. 2020. PMID: 32348766 Free PMC article.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
How RIG-I like receptors activate MAVS.Curr Opin Virol. 2015 Jun;12:91-8. doi: 10.1016/j.coviro.2015.04.004. Epub 2015 May 13. Curr Opin Virol. 2015. PMID: 25942693 Free PMC article. Review.
Cited by
-
RUNX3 inhibits KSHV lytic replication by binding to the viral genome and repressing transcription.J Virol. 2024 Feb 20;98(2):e0156723. doi: 10.1128/jvi.01567-23. Epub 2024 Jan 10. J Virol. 2024. PMID: 38197631 Free PMC article.
-
Mitochondrial protein, TBRG4, modulates KSHV and EBV reactivation from latency.PLoS Pathog. 2022 Nov 23;18(11):e1010990. doi: 10.1371/journal.ppat.1010990. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36417478 Free PMC article.
-
The herpesvirus accessory protein γ134.5 facilitates viral replication by disabling mitochondrial translocation of RIG-I.PLoS Pathog. 2021 Mar 26;17(3):e1009446. doi: 10.1371/journal.ppat.1009446. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33770145 Free PMC article.
-
RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection.Nat Commun. 2018 Nov 19;9(1):4841. doi: 10.1038/s41467-018-07314-7. Nat Commun. 2018. PMID: 30451863 Free PMC article.
-
The RLR intrinsic antiviral system is expressed in neural retina and restricts lentiviral transduction of human Mueller cells.Exp Eye Res. 2023 Nov;236:109647. doi: 10.1016/j.exer.2023.109647. Epub 2023 Sep 7. Exp Eye Res. 2023. PMID: 37689341 Free PMC article.
References
-
- Monini P, Colombini S, Sturzl M, Goletti D, Cafaro A, Sgadari C, Butto S, Franco M, Leone P, Fais S, Melucci-Vigo G, Chiozzini C, Carlini F, Ascherl G, Cornali E, Zietz C, Ramazzotti E, Ensoli F, Andreoni M, Pezzotti P, Rezza G, Yarchoan R, Gallo RC, Ensoli B. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93:4044–4058 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE018281/DE/NIDCR NIH HHS/United States
- CA109232/CA/NCI NIH HHS/United States
- AI109965/AI/NIAID NIH HHS/United States
- 1F32AI078735/AI/NIAID NIH HHS/United States
- 5T32-CA09156/CA/NCI NIH HHS/United States
- U19 AI107810/AI/NIAID NIH HHS/United States
- R01 CA109232/CA/NCI NIH HHS/United States
- 5T32-AI007151/AI/NIAID NIH HHS/United States
- T32 AI007151/AI/NIAID NIH HHS/United States
- DE018304/DE/NIDCR NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- AI107810/AI/NIAID NIH HHS/United States
- U19 AI109965/AI/NIAID NIH HHS/United States
- R01 DE018304/DE/NIDCR NIH HHS/United States
- R01 DE018281/DE/NIDCR NIH HHS/United States
- F32 AI078735/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

