HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat
- PMID: 24620025
- PMCID: PMC4148603
- DOI: 10.1093/infdis/jiu155
HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat
Abstract
Background: A single dose of the histone deacetylase inhibitor vorinostat (VOR) up-regulates HIV RNA expression within resting CD4(+) T cells of treated, aviremic human immunodeficiency virus (HIV)-positive participants. The ability of multiple exposures to VOR to repeatedly disrupt latency has not been directly measured, to our knowledge.
Methods: Five participants in whom resting CD4(+) T-cell-associated HIV RNA (rc-RNA) increased after a single dose of VOR agreed to receive daily VOR Monday through Wednesday for 8 weekly cycles. VOR serum levels, peripheral blood mononuclear cell histone acetylation, plasma HIV RNA single-copy assays, rc-RNA, total cellular HIV DNA, and quantitative viral outgrowth assays from resting CD4(+) T cells were assayed.
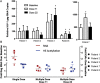
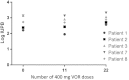
Results: VOR was well tolerated, with exposures within expected parameters. However, rc-RNA measured after dose 11 (second dose of cycle 4) or dose 22 (second dose of cycle 8) increased significantly in only 3 of the 5 participants, and the magnitude of the rc-RNA increase was much reduced compared with that after a single dose. Changes in histone acetylation were blunted. Results of quantitative viral outgrowth and other assays were unchanged.
Conclusions: Although HIV latency is disrupted by an initial VOR dose, the effect of subsequent doses in this protocol was much reduced. We hypothesize that the global effect of VOR results in a refractory period of ≥ 24 hours. The optimal schedule for VOR administration is still to be defined.
Keywords: HDAC inhibitor; HIV; acetylation; histone; latency; vorinostat.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Cellular Gene Modulation of HIV-Infected CD4 T Cells in Response to Serial Treatment with the Histone Deacetylase Inhibitor Vorinostat.J Virol. 2020 Jun 16;94(13):e00351-20. doi: 10.1128/JVI.00351-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295913 Free PMC article.
-
Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency.J Clin Invest. 2017 Aug 1;127(8):3126-3135. doi: 10.1172/JCI92684. Epub 2017 Jul 17. J Clin Invest. 2017. PMID: 28714868 Free PMC article.
-
Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy.Nature. 2012 Jul 25;487(7408):482-5. doi: 10.1038/nature11286. Nature. 2012. PMID: 22837004 Free PMC article.
-
Repurposing Vorinostat for the Treatment of Disorders Affecting Brain.Neuromolecular Med. 2021 Dec;23(4):449-465. doi: 10.1007/s12017-021-08660-4. Epub 2021 May 4. Neuromolecular Med. 2021. PMID: 33948878 Review.
-
Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir.Mol Med. 2011 May-Jun;17(5-6):466-72. doi: 10.2119/molmed.2011.00076. Epub 2011 Mar 15. Mol Med. 2011. PMID: 21424110 Free PMC article. Review.
Cited by
-
Ongoing Clinical Trials of Human Immunodeficiency Virus Latency-Reversing and Immunomodulatory Agents.Open Forum Infect Dis. 2016 Oct 7;3(4):ofw189. doi: 10.1093/ofid/ofw189. eCollection 2016 Oct. Open Forum Infect Dis. 2016. PMID: 27757411 Free PMC article. Review.
-
HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days--Implications for HIV Remission.PLoS Pathog. 2015 Jul 2;11(7):e1005000. doi: 10.1371/journal.ppat.1005000. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26133551 Free PMC article.
-
Impact of Tamoxifen on Vorinostat-Induced Human Immunodeficiency Virus Expression in Women on Antiretroviral Therapy: AIDS Clinical Trials Group A5366, The MOXIE Trial.Clin Infect Dis. 2022 Oct 12;75(8):1389-1396. doi: 10.1093/cid/ciac136. Clin Infect Dis. 2022. PMID: 35176755 Free PMC article. Clinical Trial.
-
Fighting HIV-1 Persistence: At the Crossroads of "Shoc-K and B-Lock".Pathogens. 2021 Nov 20;10(11):1517. doi: 10.3390/pathogens10111517. Pathogens. 2021. PMID: 34832672 Free PMC article. Review.
-
In vivo Effects of Romidepsin on T-Cell Activation, Apoptosis and Function in the BCN02 HIV-1 Kick&Kill Clinical Trial.Front Immunol. 2020 Mar 20;11:418. doi: 10.3389/fimmu.2020.00418. eCollection 2020. Front Immunol. 2020. PMID: 32265913 Free PMC article. Clinical Trial.
References
-
- Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–9. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. - PubMed
-
- Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. - PubMed
-
- Margolis DM. Mechanisms of HIV latency: an emerging picture of complexity. Curr HIV/AIDS Rep. 2010;7:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

