Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling
- PMID: 24586164
- PMCID: PMC3930590
- DOI: 10.1371/journal.ppat.1003960
Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling
Abstract
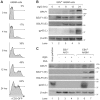
Viral infection triggers an early host response through activation of pattern recognition receptors, including Toll-like receptors (TLR). TLR signaling cascades induce production of type I interferons and proinflammatory cytokines involved in establishing an anti-viral state as well as in orchestrating ensuing adaptive immunity. To allow infection, replication, and persistence, (herpes)viruses employ ingenious strategies to evade host immunity. The human gamma-herpesvirus Epstein-Barr virus (EBV) is a large, enveloped DNA virus persistently carried by more than 90% of adults worldwide. It is the causative agent of infectious mononucleosis and is associated with several malignant tumors. EBV activates TLRs, including TLR2, TLR3, and TLR9. Interestingly, both the expression of and signaling by TLRs is attenuated during productive EBV infection. Ubiquitination plays an important role in regulating TLR signaling and is controlled by ubiquitin ligases and deubiquitinases (DUBs). The EBV genome encodes three proteins reported to exert in vitro deubiquitinase activity. Using active site-directed probes, we show that one of these putative DUBs, the conserved herpesvirus large tegument protein BPLF1, acts as a functional DUB in EBV-producing B cells. The BPLF1 enzyme is expressed during the late phase of lytic EBV infection and is incorporated into viral particles. The N-terminal part of the large BPLF1 protein contains the catalytic site for DUB activity and suppresses TLR-mediated activation of NF-κB at, or downstream of, the TRAF6 signaling intermediate. A catalytically inactive mutant of this EBV protein did not reduce NF-κB activation, indicating that DUB activity is essential for attenuating TLR signal transduction. Our combined results show that EBV employs deubiquitination of signaling intermediates in the TLR cascade as a mechanism to counteract innate anti-viral immunity of infected hosts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1.PLoS Pathog. 2023 Feb 21;19(2):e1011186. doi: 10.1371/journal.ppat.1011186. eCollection 2023 Feb. PLoS Pathog. 2023. PMID: 36802409 Free PMC article.
-
The Translesion Polymerase Pol η Is Required for Efficient Epstein-Barr Virus Infectivity and Is Regulated by the Viral Deubiquitinating Enzyme BPLF1.J Virol. 2017 Sep 12;91(19):e00600-17. doi: 10.1128/JVI.00600-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724765 Free PMC article.
-
Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-κB signaling during productive replication.J Virol. 2013 Apr;87(7):4060-70. doi: 10.1128/JVI.02020-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365429 Free PMC article.
-
[Regulation and evasion of host immune responses by Epstein-Barr virus].Wei Sheng Wu Xue Bao. 2016 Jan 4;56(1):19-25. Wei Sheng Wu Xue Bao. 2016. PMID: 27305776 Review. Chinese.
-
Immune Evasion by Epstein-Barr Virus.Curr Top Microbiol Immunol. 2015;391:355-81. doi: 10.1007/978-3-319-22834-1_12. Curr Top Microbiol Immunol. 2015. PMID: 26428381 Review.
Cited by
-
The Epstein-Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy.Autophagy. 2021 Nov;17(11):3461-3474. doi: 10.1080/15548627.2021.1874660. Epub 2021 Jan 28. Autophagy. 2021. PMID: 33509017 Free PMC article.
-
Epstein-Barr Virus and Systemic Autoimmune Diseases.Front Immunol. 2021 Jan 7;11:587380. doi: 10.3389/fimmu.2020.587380. eCollection 2020. Front Immunol. 2021. PMID: 33488588 Free PMC article. Review.
-
Epstein-Barr virus: Biology and clinical disease.Cell. 2022 Sep 29;185(20):3652-3670. doi: 10.1016/j.cell.2022.08.026. Epub 2022 Sep 15. Cell. 2022. PMID: 36113467 Free PMC article. Review.
-
Viral Ubiquitin and Ubiquitin-Like Deconjugases-Swiss Army Knives for Infection.Biomolecules. 2020 Aug 1;10(8):1137. doi: 10.3390/biom10081137. Biomolecules. 2020. PMID: 32752270 Free PMC article. Review.
-
Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1.PLoS Pathog. 2023 Feb 21;19(2):e1011186. doi: 10.1371/journal.ppat.1011186. eCollection 2023 Feb. PLoS Pathog. 2023. PMID: 36802409 Free PMC article.
References
-
- Rickinson AB, Kieff E (2007) Epstein-Barr Virus. In: Knipe DM, Howley PM, editors. Field's Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 2655–2700.
-
- Kutok JL, Wang F (2006) Spectrum of Epstein-Barr Virus-Associated Diseases. Annu Rev Pathol Mech Dis 1: 375–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

