Effects of anti-g and anti-f antibodies on airway function after respiratory syncytial virus infection
- PMID: 24521403
- PMCID: PMC4091856
- DOI: 10.1165/rcmb.2013-0360OC
Effects of anti-g and anti-f antibodies on airway function after respiratory syncytial virus infection
Abstract
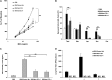
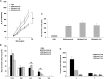
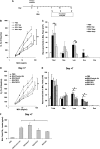
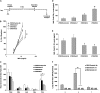
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract illnesses in infants worldwide. Both RSV-G and RSV-F glycoproteins play pathogenic roles during infection with RSV. The objective of this study was to compare the effects of anti-RSV-G and anti-RSV-F monoclonal antibodies (mAbs) on airway hyperresponsiveness (AHR) and inflammation after primary or secondary RSV infection in mice. In the primary infection model, mice were infected with RSV at 6 weeks of age. Anti-RSV-G or anti-RSV-F mAbs were administered 24 hours before infection or Day +2 postinfection. In a secondary infection model, mice were infected (primary) with RSV at 1 week (neonate) and reinfected (secondary) 5 weeks later. Anti-RSV-G and anti-RSV-F mAbs were administered 24 hours before the primary infection. Both mAbs had comparable effects in preventing airway responses after primary RSV infection. When given 2 days after infection, anti-RSV-G-treated mice showed significantly decreased AHR and airway inflammation, which persisted in anti-RSV-F-treated mice. In the reinfection model, anti-RSV-G but not anti-RSV-F administered during primary RSV infection in neonates resulted in decreased AHR, eosinophilia, and IL-13 but increased levels of IFN-γ in bronchoalveolar lavage on reinfection. These results support the use of anti-RSV-G in the prevention and treatment of RSV-induced disease.
Keywords: airway; anti–respiratory syncytial virus–F; anti–respiratory syncytial virus–G; inflammation; respiratory syncytial virus.
Figures

Similar articles
-
Montelukast during primary infection prevents airway hyperresponsiveness and inflammation after reinfection with respiratory syncytial virus.Am J Respir Crit Care Med. 2010 Aug 15;182(4):455-63. doi: 10.1164/rccm.200912-1811OC. Epub 2010 May 4. Am J Respir Crit Care Med. 2010. PMID: 20442434 Free PMC article.
-
Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice.J Virol. 2010 Sep;84(18):9632-6. doi: 10.1128/JVI.00451-10. Epub 2010 Jun 30. J Virol. 2010. PMID: 20592094 Free PMC article.
-
Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice.J Allergy Clin Immunol. 2009 Jan;123(1):138-145.e5. doi: 10.1016/j.jaci.2008.10.012. Epub 2008 Dec 3. J Allergy Clin Immunol. 2009. PMID: 19056111
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
-
[Research Progress in the F Gene and Protein of the Respiratory Syncytial Virus].Bing Du Xue Bao. 2015 Mar;31(2):201-6. Bing Du Xue Bao. 2015. PMID: 26164949 Review. Chinese.
Cited by
-
Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies.PLoS Pathog. 2018 Mar 6;14(3):e1006935. doi: 10.1371/journal.ppat.1006935. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29509814 Free PMC article.
-
Immune Prophylaxis Targeting the Respiratory Syncytial Virus (RSV) G Protein.Viruses. 2023 Apr 27;15(5):1067. doi: 10.3390/v15051067. Viruses. 2023. PMID: 37243153 Free PMC article.
-
Enhancing Anti-G Antibody Induction by a Live Single-Cycle Prefusion F-Expressing RSV Vaccine Improves In Vitro and In Vivo Efficacy.Viruses. 2022 Nov 9;14(11):2474. doi: 10.3390/v14112474. Viruses. 2022. PMID: 36366572 Free PMC article.
-
Respiratory Syncytial Virus (RSV) G Protein Vaccines With Central Conserved Domain Mutations Induce CX3C-CX3CR1 Blocking Antibodies.Viruses. 2021 Feb 23;13(2):352. doi: 10.3390/v13020352. Viruses. 2021. PMID: 33672319 Free PMC article.
-
Upregulation of CD32 in T Cells from Infants with Severe Respiratory Syncytial Virus Disease: A New Costimulatory Pathway?Am J Respir Cell Mol Biol. 2020 Jul;63(1):133-136. doi: 10.1165/rcmb.2020-0025LE. Am J Respir Cell Mol Biol. 2020. PMID: 32609012 Free PMC article. No abstract available.
References
-
- Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

