B cells mediate chronic allograft rejection independently of antibody production
- PMID: 24509079
- PMCID: PMC3934170
- DOI: 10.1172/JCI70084
B cells mediate chronic allograft rejection independently of antibody production
Abstract
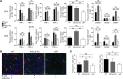
Chronic rejection is the primary cause of long-term failure of transplanted organs and is often viewed as an antibody-dependent process. Chronic rejection, however, is also observed in mice and humans with no detectable circulating alloantibodies, suggesting that antibody-independent pathways may also contribute to pathogenesis of transplant rejection. Here, we have provided direct evidence that chronic rejection of vascularized heart allografts occurs in the complete absence of antibodies, but requires the presence of B cells. Mice that were deficient for antibodies but not B cells experienced the same chronic allograft vasculopathy (CAV), which is a pathognomonic feature of chronic rejection, as WT mice; however, mice that were deficient for both B cells and antibodies were protected from CAV. B cells contributed to CAV by supporting splenic lymphoid architecture, T cell cytokine production, and infiltration of T cells into graft vessels. In chimeric mice, in which B cells were present but could not present antigen, both T cell responses and CAV were markedly reduced. These findings establish that chronic rejection can occur in the complete absence of antibodies and that B cells contribute to this process by supporting T cell responses through antigen presentation and maintenance of lymphoid architecture.
Figures
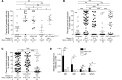

Similar articles
-
Natural killer cells play a critical role in cardiac allograft vasculopathy in an interleukin-6--dependent manner.Transplantation. 2014 Nov 27;98(10):1029-39. doi: 10.1097/TP.0000000000000405. Transplantation. 2014. PMID: 25286056
-
Interleukin-6 receptor signaling disruption prevents cardiac allograft deterioration in mice.Exp Clin Transplant. 2012 Aug;10(4):375-85. doi: 10.6002/ect.2011.0159. Epub 2012 Jul 2. Exp Clin Transplant. 2012. PMID: 22758208
-
B cells assist allograft rejection in the deficiency of protein kinase c-theta.Transpl Int. 2013 Sep;26(9):919-27. doi: 10.1111/tri.12143. Epub 2013 Jul 11. Transpl Int. 2013. PMID: 23841454
-
B cells as antigen-presenting cells in transplantation rejection and tolerance.Cell Immunol. 2020 Mar;349:104061. doi: 10.1016/j.cellimm.2020.104061. Epub 2020 Feb 7. Cell Immunol. 2020. PMID: 32059816 Free PMC article. Review.
-
Effector B cells in cardiac allograft vasculopathy.Curr Opin Organ Transplant. 2019 Feb;24(1):31-36. doi: 10.1097/MOT.0000000000000591. Curr Opin Organ Transplant. 2019. PMID: 30480642 Free PMC article. Review.
Cited by
-
Recent advances in renal transplantation: antibody-mediated rejection takes center stage.F1000Prime Rep. 2015 May 12;7:51. doi: 10.12703/P7-51. eCollection 2015. F1000Prime Rep. 2015. PMID: 26097724 Free PMC article. Review.
-
Mitochondrial regulation of acute extrafollicular B-cell responses to COVID-19 severity.Clin Transl Med. 2022 Sep;12(9):e1025. doi: 10.1002/ctm2.1025. Clin Transl Med. 2022. PMID: 36103567 Free PMC article.
-
Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity.Cell Rep Med. 2022 Sep 20;3(9):100744. doi: 10.1016/j.xcrm.2022.100744. Epub 2022 Sep 12. Cell Rep Med. 2022. PMID: 36099917 Free PMC article.
-
Hepatic Stellate Cells Directly Inhibit B Cells via Programmed Death-Ligand 1.J Immunol. 2016 Feb 15;196(4):1617-25. doi: 10.4049/jimmunol.1501737. Epub 2016 Jan 11. J Immunol. 2016. PMID: 26755818 Free PMC article.
-
A minor tweak in transplant surgery protocols alters the cellular landscape of the arterial wall during transplant vasculopathy.Front Transplant. 2024 Apr 29;3:1260125. doi: 10.3389/frtra.2024.1260125. eCollection 2024. Front Transplant. 2024. PMID: 38993774 Free PMC article.
References
-
- Radio S, Wood S, Wilson J, Lin H, Winters G, McManus B. Allograft vascular disease: comparison of heart and other grafted organs. Transplant Proc. 1996;28(1):496–499. - PubMed
-
- Mauiyyedi S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12(3):574–582. - PubMed
-
- Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86(3):377–383. - PubMed
-
- Nath DS, et al. Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major histocompatibility complex class I-related chain A in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol. 2010;71(12):1191–1196. doi: 10.1016/j.humimm.2010.09.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

