Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species
- PMID: 24424030
- PMCID: PMC3953816
- DOI: 10.1242/jcs.140087
Differential roles of the ubiquitin proteasome system and autophagy in the clearance of soluble and aggregated TDP-43 species
Abstract
TAR DNA-binding protein (TDP-43, also known as TARDBP) is the major pathological protein in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Large TDP-43 aggregates that are decorated with degradation adaptor proteins are seen in the cytoplasm of remaining neurons in ALS and FTD patients post mortem. TDP-43 accumulation and ALS-linked mutations within degradation pathways implicate failed TDP-43 clearance as a primary disease mechanism. Here, we report the differing roles of the ubiquitin proteasome system (UPS) and autophagy in the clearance of TDP-43. We have investigated the effects of inhibitors of the UPS and autophagy on the degradation, localisation and mobility of soluble and insoluble TDP-43. We find that soluble TDP-43 is degraded primarily by the UPS, whereas the clearance of aggregated TDP-43 requires autophagy. Cellular macroaggregates, which recapitulate many of the pathological features of the aggregates in patients, are reversible when both the UPS and autophagy are functional. Their clearance involves the autophagic removal of oligomeric TDP-43. We speculate that, in addition to an age-related decline in pathway activity, a second hit in either the UPS or the autophagy pathway drives the accumulation of TDP-43 in ALS and FTD. Therapies for clearing excess TDP-43 should therefore target a combination of these pathways.
Keywords: ALS; Aggrephagy; Autophagy; Proteasome; TDP-43; UPS.
Figures
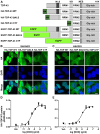

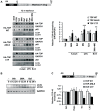
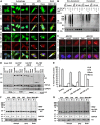
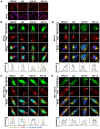
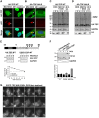
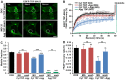
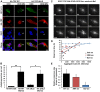

Similar articles
-
Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs.J Vis Exp. 2023 May 26;(195). doi: 10.3791/6561. J Vis Exp. 2023. PMID: 37235796
-
Distribution of ubiquilin 2 and TDP-43 aggregates throughout the CNS in UBQLN2 p.T487I-linked amyotrophic lateral sclerosis and frontotemporal dementia.Brain Pathol. 2024 May;34(3):e13230. doi: 10.1111/bpa.13230. Epub 2023 Dec 19. Brain Pathol. 2024. PMID: 38115557 Free PMC article.
-
Tar DNA-binding protein-43 (TDP-43) regulates axon growth in vitro and in vivo.Neurobiol Dis. 2014 May;65(100):25-34. doi: 10.1016/j.nbd.2014.01.004. Epub 2014 Jan 11. Neurobiol Dis. 2014. PMID: 24423647 Free PMC article.
-
TARDBP-Related Amyotrophic Lateral Sclerosis-Frontotemporal Dementia.2009 Apr 23 [updated 2023 Jan 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2009 Apr 23 [updated 2023 Jan 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301761 Free Books & Documents. Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
-
Experimental Disease-Modifying Agents for Frontotemporal Lobar Degeneration.J Exp Pharmacol. 2021 Mar 24;13:359-376. doi: 10.2147/JEP.S262352. eCollection 2021. J Exp Pharmacol. 2021. PMID: 33790662 Free PMC article. Review.
-
Transthyretin attenuates TDP-43 proteinopathy by autophagy activation via ATF4 in FTLD-TDP.Brain. 2023 May 2;146(5):2089-2106. doi: 10.1093/brain/awac412. Brain. 2023. PMID: 36355566 Free PMC article.
-
ALS' Perfect Storm: C9orf72-Associated Toxic Dipeptide Repeats as Potential Multipotent Disruptors of Protein Homeostasis.Cells. 2024 Jan 17;13(2):178. doi: 10.3390/cells13020178. Cells. 2024. PMID: 38247869 Free PMC article. Review.
-
ALS-associated missense and nonsense TBK1 mutations can both cause loss of kinase function.Neurobiol Aging. 2018 Nov;71:266.e1-266.e10. doi: 10.1016/j.neurobiolaging.2018.06.015. Epub 2018 Jun 25. Neurobiol Aging. 2018. PMID: 30033073 Free PMC article.
-
Mechanisms underlying TDP-43 pathology and neurodegeneration: An updated Mini-Review.Front Aging Neurosci. 2023 Mar 9;15:1142617. doi: 10.3389/fnagi.2023.1142617. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36967829 Free PMC article. Review.
References
-
- Arai T., Nonaka T., Hasegawa M., Akiyama H., Yoshida M., Hashizume Y., Tsuchiya K., Oda T., Ikeda K. (2003). Neuronal and glial inclusions in frontotemporal dementia with or without motor neuron disease are immunopositive for p62. Neurosci. Lett. 342, 41–44 10.1016/S0304-3940(03)00216-7 - DOI - PubMed
-
- Bigio E. H., Wu J. Y., Deng H. X., Bit-Ivan E. N., Mao Q., Ganti R., Peterson M., Siddique N., Geula C., Siddique T. et al. (2013). Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta Neuropathol. 125, 463–465 10.1007/s00401-013-1089-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 089701/WT_/Wellcome Trust/United Kingdom
- G0501573/MRC_/Medical Research Council/United Kingdom
- G0900635/MRC_/Medical Research Council/United Kingdom
- 089703/WT_/Wellcome Trust/United Kingdom
- MC_G1000734/MRC_/Medical Research Council/United Kingdom
- G0600974/MRC_/Medical Research Council/United Kingdom
- G1100695/MRC_/Medical Research Council/United Kingdom
- 089701/MRC_/Medical Research Council/United Kingdom
- MC_G1000733/MRC_/Medical Research Council/United Kingdom
- G0500289/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 095317/WT_/Wellcome Trust/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- G0900688/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

