Where is mTOR and what is it doing there?
- PMID: 24385483
- PMCID: PMC3840941
- DOI: 10.1083/jcb.201306041
Where is mTOR and what is it doing there?
Abstract
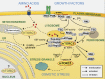
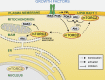
Target of rapamycin (TOR) forms two conserved, structurally distinct kinase complexes termed TOR complex 1 (TORC1) and TORC2. Each complex phosphorylates a different set of substrates to regulate cell growth. In mammals, mTOR is stimulated by nutrients and growth factors and inhibited by stress to ensure that cells grow only during favorable conditions. Studies in different organisms have reported localization of TOR to several distinct subcellular compartments. Notably, the finding that mTORC1 is localized to the lysosome has significantly enhanced our understanding of mTORC1 regulation. Subcellular localization may be a general principle used by TOR to enact precise spatial and temporal control of cell growth.
Figures
Similar articles
-
mTOR substrate phosphorylation in growth control.Cell. 2022 May 26;185(11):1814-1836. doi: 10.1016/j.cell.2022.04.013. Epub 2022 May 16. Cell. 2022. PMID: 35580586 Review.
-
Transforming growth factor β integrates Smad 3 to mechanistic target of rapamycin complexes to arrest deptor abundance for glomerular mesangial cell hypertrophy.J Biol Chem. 2013 Mar 15;288(11):7756-7768. doi: 10.1074/jbc.M113.455782. Epub 2013 Jan 28. J Biol Chem. 2013. PMID: 23362262 Free PMC article.
-
Nutrient signaling to mTOR and cell growth.Trends Biochem Sci. 2013 May;38(5):233-42. doi: 10.1016/j.tibs.2013.01.004. Epub 2013 Mar 1. Trends Biochem Sci. 2013. PMID: 23465396 Free PMC article. Review.
-
TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing.J Cell Sci. 2012 Jan 1;125(Pt 1):37-48. doi: 10.1242/jcs.077040. Epub 2012 Jan 20. J Cell Sci. 2012. PMID: 22266904 Free PMC article.
-
RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells.Cell Signal. 2014 Mar;26(3):461-7. doi: 10.1016/j.cellsig.2013.11.035. Epub 2013 Dec 3. Cell Signal. 2014. PMID: 24316235 Free PMC article.
Cited by
-
Regulation of mTORC1 by Upstream Stimuli.Genes (Basel). 2020 Aug 25;11(9):989. doi: 10.3390/genes11090989. Genes (Basel). 2020. PMID: 32854217 Free PMC article. Review.
-
Transient activation of mTORC1 signaling in skeletal muscle is independent of Akt1 regulation.Physiol Rep. 2020 Oct;8(19):e14599. doi: 10.14814/phy2.14599. Physiol Rep. 2020. PMID: 33038070 Free PMC article.
-
siRNA Blocking of Mammalian Target of Rapamycin (mTOR) Attenuates Pathology in Annonacin-Induced Tauopathy in Mice.Neurotox Res. 2019 May;35(4):987-992. doi: 10.1007/s12640-018-9974-3. Epub 2018 Oct 25. Neurotox Res. 2019. PMID: 30362086
-
Rapid manipulation of mitochondrial morphology in a living cell with iCMM.Cell Rep Methods. 2021 Jul 22;1(4):100052. doi: 10.1016/j.crmeth.2021.100052. eCollection 2021 Aug 23. Cell Rep Methods. 2021. PMID: 35475143 Free PMC article.
-
mTORC2: a multifaceted regulator of autophagy.Cell Commun Signal. 2023 Jan 5;21(1):4. doi: 10.1186/s12964-022-00859-7. Cell Commun Signal. 2023. PMID: 36604720 Free PMC article. Review.
References
-
- Aronova S., Wedaman K., Anderson S., Yates J., III, Powers T.. 2007. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol. Biol. Cell. 18:2779–2794. 10.1091/mbc.E07-03-0274 - DOI - PMC - PubMed
-
- Aslan J.E., You H., Williamson D.M., Endig J., Youker R.T., Thomas L., Shu H., Du Y., Milewski R.L., Brush M.H., et al. . 2009. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol. Cell. 34:497–509. 10.1016/j.molcel.2009.04.011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

