Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via suppressing iNOS expression and the CTGF (CCN2)/ERK signaling pathway
- PMID: 24351828
- PMCID: PMC3876122
- DOI: 10.3390/ijms141224476
Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via suppressing iNOS expression and the CTGF (CCN2)/ERK signaling pathway
Abstract
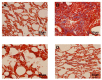
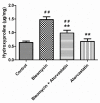
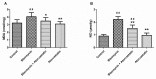
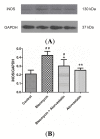
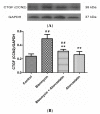
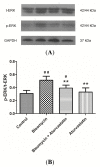
Pulmonary fibrosis is a progressive and fatal lung disorder with high mortality rate. To date, despite the fact that extensive research trials are ongoing, pulmonary fibrosis continues to have a poor response to available medical therapy. Statins, 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, known for its broad pharmacological activities, remains a remedy against multiple diseases. The present study investigated the antifibrotic potential of atorvastatin against bleomycin-induced lung fibrosis and to further explore the possible underlying mechanisms. Our results showed that atorvastatin administration significantly ameliorated the bleomycin mediated histological alterations and blocked collagen deposition with parallel reduction in the hydroxyproline level. Atorvastatin reduced malondialdehyde (MDA) level and lung indices. Atorvastatin also markedly decreased the expression of inducible nitric oxide synthase (iNOS) in lung tissues and, thus, prevented nitric oxide (NO) release in response to bleomycin challenge. Furthermore, atorvastatin exhibited target down-regulation of connective tissue growth factor (CTGF (CCN2)) and phosphorylation extracellular regulated protein kinases (p-ERK) expression. Taken together, atorvastatin significantly ameliorated bleomycin-induced pulmonary fibrosis in rats, via the inhibition of iNOS expression and the CTGF (CCN2)/ERK signaling pathway. The present study provides evidence that atorvastatin may be a potential therapeutic reagent for the treatment of lung fibrosis.
Figures

Similar articles
-
Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-κB dependant TGF-β activation: a biphasic experimental study.Toxicol Lett. 2013 May 23;219(2):178-93. doi: 10.1016/j.toxlet.2013.03.009. Epub 2013 Mar 21. Toxicol Lett. 2013. PMID: 23523906
-
A Chinese Traditional Therapy for Bleomycin-Induced Pulmonary Fibrosis in Mice.Can Respir J. 2018 Sep 18;2018:8491487. doi: 10.1155/2018/8491487. eCollection 2018. Can Respir J. 2018. PMID: 30319721 Free PMC article.
-
Atorvastatin attenuates mitochondrial toxin-induced striatal degeneration, with decreasing iNOS/c-Jun levels and activating ERK/Akt pathways.J Neurochem. 2008 Mar;104(5):1190-200. doi: 10.1111/j.1471-4159.2007.05044.x. Epub 2007 Nov 1. J Neurochem. 2008. PMID: 17976163
-
Atorvastatin: pharmacological characteristics and lipid-lowering effects.Drugs. 2007;67 Suppl 1:3-15. doi: 10.2165/00003495-200767001-00002. Drugs. 2007. PMID: 17910517 Review.
-
Drugs targeting CTGF in the treatment of pulmonary fibrosis.J Cell Mol Med. 2024 May;28(10):e18448. doi: 10.1111/jcmm.18448. J Cell Mol Med. 2024. PMID: 38774993 Free PMC article. Review.
Cited by
-
Extracellular Lipids in the Lung and Their Role in Pulmonary Fibrosis.Cells. 2022 Apr 3;11(7):1209. doi: 10.3390/cells11071209. Cells. 2022. PMID: 35406772 Free PMC article. Review.
-
Therapeutic effect of adipose-derived mesenchymal stem cells (AD-MSCs) compared to pirfenidone on corticosteroid resistance in a mouse model of acute exacerbation of idiopathic pulmonary fibrosis.Histol Histopathol. 2022 Nov;37(11):1065-1083. doi: 10.14670/HH-18-493. Epub 2022 Jul 11. Histol Histopathol. 2022. PMID: 35816024
-
LDLR dysfunction induces LDL accumulation and promotes pulmonary fibrosis.Clin Transl Med. 2022 Jan;12(1):e711. doi: 10.1002/ctm2.711. Clin Transl Med. 2022. PMID: 35083881 Free PMC article.
-
Rho inhibition by lovastatin affects apoptosis and DSB repair of primary human lung cells in vitro and lung tissue in vivo following fractionated irradiation.Cell Death Dis. 2017 Aug 10;8(8):e2978. doi: 10.1038/cddis.2017.372. Cell Death Dis. 2017. PMID: 28796249 Free PMC article.
-
Lung Pneumonitis and Fibrosis in Cancer Therapy: A Review on Cellular and Molecular Mechanisms.Curr Drug Targets. 2022;23(16):1505-1525. doi: 10.2174/1389450123666220907144131. Curr Drug Targets. 2022. PMID: 36082868 Review.
References
-
- Selman M., Thannickal V.J., Pardo A., Zisman D.A., Martinez F.J., Lynch J.P., 3rd Idiopathic pulmonary fibrosis: Pathogenesis and therapeutic approaches. Drugs. 2004;64:405–430. - PubMed
-
- Raghu G., Chang J. Idiopathic pulmonary fibrosis: Current trends in management. Clin. Chest Med. 2004;25:621–636. - PubMed
-
- Maher T.M. Idiopathic pulmonary fibrosis: Pathobiology of novel approaches to treatment. Clin. Chest Med. 2012;33:69–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

