Maraba virus as a potent oncolytic vaccine vector
- PMID: 24322333
- PMCID: PMC3916044
- DOI: 10.1038/mt.2013.249
Maraba virus as a potent oncolytic vaccine vector
Abstract
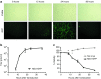
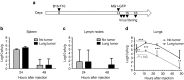
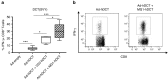
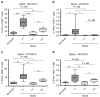
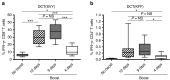
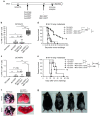
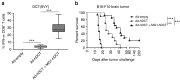
The rhabdovirus Maraba has recently been characterized as a potent oncolytic virus. In the present study, we engineered an attenuated Maraba strain, defined as MG1, to express a melanoma-associated tumor antigen. Its ability to mount an antitumor immunity was evaluated in tumor-free and melanoma tumor-bearing mice. Alone, the MG1 vaccine appeared insufficient to prime detectable adaptive immunity against the tumor antigen. However, when used as a boosting vector in a heterologous prime-boost regimen, MG1 vaccine rapidly generated strong antigen-specific T-cell immune responses. Once applied for treating syngeneic murine melanoma tumors, our oncolytic prime-boost vaccination protocol involving Maraba MG1 dramatically extended median survival and allowed complete remission in more than 20% of the animals treated. This work describes Maraba virus MG1 as a potent vaccine vector for cancer immunotherapy displaying both oncolytic activity and a remarkable ability to boost adaptive antitumor immunity.
Figures

Similar articles
-
Considerations for Clinical Translation of MG1 Maraba Virus.Methods Mol Biol. 2020;2058:285-293. doi: 10.1007/978-1-4939-9794-7_19. Methods Mol Biol. 2020. PMID: 31486046 Review.
-
Oncolytic Maraba virus armed with tumor antigen boosts vaccine priming and reveals diverse therapeutic response patterns when combined with checkpoint blockade in ovarian cancer.J Immunother Cancer. 2019 Jul 17;7(1):189. doi: 10.1186/s40425-019-0641-x. J Immunother Cancer. 2019. PMID: 31315674 Free PMC article.
-
Brief Communication; A Heterologous Oncolytic Bacteria-Virus Prime-Boost Approach for Anticancer Vaccination in Mice.J Immunother. 2018 Apr;41(3):125-129. doi: 10.1097/CJI.0000000000000208. J Immunother. 2018. PMID: 29293165 Free PMC article.
-
Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus.Mol Ther. 2009 Oct;17(10):1814-21. doi: 10.1038/mt.2009.154. Epub 2009 Jul 14. Mol Ther. 2009. PMID: 19603003 Free PMC article.
-
Development and applications of oncolytic Maraba virus vaccines.Oncolytic Virother. 2018 Nov 26;7:117-128. doi: 10.2147/OV.S154494. eCollection 2018. Oncolytic Virother. 2018. PMID: 30538968 Free PMC article. Review.
Cited by
-
Oncolytic virus and PD-1/PD-L1 blockade combination therapy.Oncolytic Virother. 2018 Jul 31;7:65-77. doi: 10.2147/OV.S145532. eCollection 2018. Oncolytic Virother. 2018. PMID: 30105219 Free PMC article. Review.
-
Going viral with cancer immunotherapy.Nat Rev Cancer. 2014 Aug;14(8):559-67. doi: 10.1038/nrc3770. Epub 2014 Jul 3. Nat Rev Cancer. 2014. PMID: 24990523 Review.
-
Dendritic Cells in Oncolytic Virus-Based Anti-Cancer Therapy.Viruses. 2015 Dec 9;7(12):6506-25. doi: 10.3390/v7122953. Viruses. 2015. PMID: 26690204 Free PMC article. Review.
-
Activating Peripheral Innate Immunity Enables Safe and Effective Oncolytic Virotherapy in the Brain.Mol Ther Oncolytics. 2017 Sep 15;7:45-56. doi: 10.1016/j.omto.2017.09.004. eCollection 2017 Dec 15. Mol Ther Oncolytics. 2017. PMID: 29062886 Free PMC article.
-
Perspectives on immunotherapy via oncolytic viruses.Infect Agent Cancer. 2019 Feb 11;14:5. doi: 10.1186/s13027-018-0218-1. eCollection 2019. Infect Agent Cancer. 2019. PMID: 30792754 Free PMC article. Review.
References
-
- Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005–4015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

