Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation
- PMID: 24316970
- PMCID: PMC3871219
- DOI: 10.1172/JCI66947
Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation
Abstract
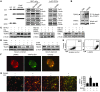
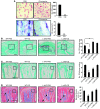
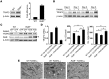
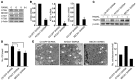
The cytokines RANKL and TNF activate NF-κB signaling in osteoclast precursors (OCPs) to induce osteoclast (OC) formation. Conversely, TNF can limit OC formation through NF-κB p100, which acts as an inhibitor, and TNF receptor-associated receptor 3 (TRAF3); however, a role for TRAF3 in RANKL-mediated OC formation is unknown. We found that TRAF3 limits RANKL-induced osteoclastogenesis by suppressing canonical and noncanonical NF-κB signaling. Conditional OC-specific Traf3-KO (cKO) mice had mild osteoporosis and increased OC formation. RANKL induced TRAF3 degradation via the lysosome/autophagy system. The autophagy/lysosome inhibitor chloroquine reduced RANKL-induced OC formation and function by increasing TRAF3 expression in OCPs in vitro and in vivo. Although chloroquine had no effect on basal bone resorption, it inhibited parathyroid hormone- and ovariectomy-induced OC activation in WT, but not cKO, mice. Deletion of the transcription factor gene Relb resulted in increased TRAF3 expression in OCPs, which was associated with decreased RANKL-induced TRAF3 degradation. RelB directly increased expression of BECN1, a key autophagy regulator, by binding to its promoter. These data indicate that autophagic/lysosomal degradation of TRAF3 is an important step in RANKL-induced NF-κB activation in OCPs. Furthermore, treatments that increase TRAF3 levels in OCPs, including pharmacological inhibition of its degradation with compounds such as chloroquine, may limit bone destruction in common bone diseases.
Figures




Similar articles
-
RANKL cytokine enhances TNF-induced osteoclastogenesis independently of TNF receptor associated factor (TRAF) 6 by degrading TRAF3 in osteoclast precursors.J Biol Chem. 2017 Jun 16;292(24):10169-10179. doi: 10.1074/jbc.M116.771816. Epub 2017 Apr 24. J Biol Chem. 2017. PMID: 28438834 Free PMC article.
-
NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism.J Clin Invest. 2009 Oct;119(10):3024-34. doi: 10.1172/JCI38716. Epub 2009 Sep 21. J Clin Invest. 2009. PMID: 19770515 Free PMC article.
-
Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption.Front Immunol. 2018 Sep 28;9:2263. doi: 10.3389/fimmu.2018.02263. eCollection 2018. Front Immunol. 2018. PMID: 30323820 Free PMC article. Review.
-
TNF Induction of NF-κB RelB Enhances RANKL-Induced Osteoclastogenesis by Promoting Inflammatory Macrophage Differentiation but also Limits It through Suppression of NFATc1 Expression.PLoS One. 2015 Aug 19;10(8):e0135728. doi: 10.1371/journal.pone.0135728. eCollection 2015. PLoS One. 2015. PMID: 26287732 Free PMC article.
-
Regulation of TNF-Induced Osteoclast Differentiation.Cells. 2021 Dec 31;11(1):132. doi: 10.3390/cells11010132. Cells. 2021. PMID: 35011694 Free PMC article. Review.
Cited by
-
Vascular Pericyte-Derived Exosomes Inhibit Bone Resorption via Traf3.Int J Nanomedicine. 2023 Nov 28;18:7065-7077. doi: 10.2147/IJN.S438229. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38046234 Free PMC article.
-
Role of autophagy in osteosarcoma.J Bone Oncol. 2019 Apr 3;16:100235. doi: 10.1016/j.jbo.2019.100235. eCollection 2019 Jun. J Bone Oncol. 2019. PMID: 31011524 Free PMC article. Review.
-
Design of Nanodrug Delivery Systems for Tumor Bone Metastasis.Curr Pharm Des. 2024;30(15):1136-1148. doi: 10.2174/0113816128296883240320040636. Curr Pharm Des. 2024. PMID: 38551047 Review.
-
The Chx10-Traf3 Knockout Mouse as a Viable Model to Study Neuronal Immune Regulation.Cells. 2021 Aug 12;10(8):2068. doi: 10.3390/cells10082068. Cells. 2021. PMID: 34440839 Free PMC article.
-
The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy.Onco Targets Ther. 2017 Jul 27;10:3801-3810. doi: 10.2147/OTT.S135867. eCollection 2017. Onco Targets Ther. 2017. PMID: 28794644 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

