Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms
- PMID: 24281006
- PMCID: PMC3887295
- DOI: 10.1038/bjc.2013.695
Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms
Abstract
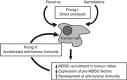
Background: Reovirus preferentially infects and kills cancer cells and is currently undergoing clinical trials internationally. While oncolysis is the primary mode of tumour elimination, increasing evidence illustrates that reovirus additionally stimulates anti-tumour immunity with a capacity to target existing and possibly relapsing cancer cells. These virus-induced anti-tumour immune activities largely determine the efficacy of oncotherapy. On the other hand, anti-viral immune responses can negatively affect oncotherapy. Hence, the strategic management of anti-tumour and anti-viral immune responses through complementary therapeutics is crucial to achieve the maximum anti-cancer benefits of oncotherapy.
Methods: Intra-peritoneal injection of mouse ovarian surface epithelial cells (ID8 cells) into wild-type C57BL/6 mice was treated with a therapeutic regimen of reovirus and/or gemcitabine and then analysed for prolonged survival, disease pathology, and various immunological parameters. Furthermore, in vitro analyses were conducted to assess apoptosis, viral spread, and viral production during reovirus and/or gemcitabine treatment.
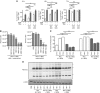
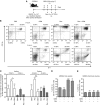
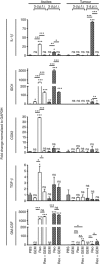
Results: We demonstrate that reovirus and gemcitabine combination treatment postpones peritoneal carcinomatosis development and prolongs the survival of cancer-bearing hosts. Importantly, these anti-cancer benefits are generated through various immunological mechanisms, including: (1) inhibition of myeloid-derived suppressor cells recruitment to the tumour microenvironment, (2) downmodulation of pro-MDSC factors, and (3) accelerated development of anti-tumour T-cell responses.
Conclusion: The complementation of reovirus with gemcitabine further potentiates virus-initiated anti-cancer immunity and enhances the efficacy of oncotherapy. In the context of ongoing clinical trials, our findings represent clinically relevant information capable of enhancing cancer outcomes.
Figures
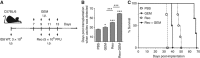


Similar articles
-
Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis.J Immunother Cancer. 2021 Mar;9(3):e002096. doi: 10.1136/jitc-2020-002096. J Immunother Cancer. 2021. PMID: 33722907 Free PMC article.
-
Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity.Gene Ther. 2009 May;16(5):689-99. doi: 10.1038/gt.2009.29. Epub 2009 Mar 12. Gene Ther. 2009. PMID: 19282847 Free PMC article.
-
Oncolytic adenovirus Ad5/3-delta24 and chemotherapy for treatment of orthotopic ovarian cancer.Gynecol Oncol. 2008 Jan;108(1):166-72. doi: 10.1016/j.ygyno.2007.09.013. Epub 2007 Oct 22. Gynecol Oncol. 2008. PMID: 17950450
-
Oncolytic viral therapy using reovirus.Methods Mol Biol. 2009;542:607-34. doi: 10.1007/978-1-59745-561-9_31. Methods Mol Biol. 2009. PMID: 19565924 Review.
-
Advances in oncolytic adenovirus therapy for pancreatic cancer.Cancer Lett. 2018 Oct 10;434:56-69. doi: 10.1016/j.canlet.2018.07.006. Epub 2018 Jul 5. Cancer Lett. 2018. PMID: 29981812 Review.
Cited by
-
Colonization of xenograft tumors by oncolytic vaccinia virus (VACV) results in enhanced tumor killing due to the involvement of myeloid cells.J Transl Med. 2016 Dec 20;14(1):340. doi: 10.1186/s12967-016-1096-1. J Transl Med. 2016. PMID: 27993141 Free PMC article.
-
Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity.World J Methodol. 2016 Mar 26;6(1):25-42. doi: 10.5662/wjm.v6.i1.25. eCollection 2016 Mar 26. World J Methodol. 2016. PMID: 27019795 Free PMC article. Review.
-
Parvovirus-Based Combinatorial Immunotherapy: A Reinforced Therapeutic Strategy against Poor-Prognosis Solid Cancers.Cancers (Basel). 2021 Jan 19;13(2):342. doi: 10.3390/cancers13020342. Cancers (Basel). 2021. PMID: 33477757 Free PMC article. Review.
-
Natural Killer Cell Interactions With Myeloid Derived Suppressor Cells in the Tumor Microenvironment and Implications for Cancer Immunotherapy.Front Immunol. 2021 May 5;12:633205. doi: 10.3389/fimmu.2021.633205. eCollection 2021. Front Immunol. 2021. PMID: 34025641 Free PMC article. Review.
-
Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances.Oncolytic Virother. 2016 Jan 6;5:1-13. doi: 10.2147/OV.S66083. eCollection 2016. Oncolytic Virother. 2016. PMID: 27579292 Free PMC article. Review.
References
-
- Bellmunt J, von der MH, Mead GM, Skoneczna I, De SM, Daugaard G, Boehle A, Chevreau C, Paz-Ares L, Laufman LR, Winquist E, Raghavan D, Marreaud S, Collette S, Sylvester R, de Wit R. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30 (10:1107–1113. - PMC - PubMed
-
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176 (1:284–290. - PubMed
-
- Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282 (5392:1332–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

