Keeping it quiet: chromatin control of gammaherpesvirus latency
- PMID: 24192651
- PMCID: PMC4544771
- DOI: 10.1038/nrmicro3135
Keeping it quiet: chromatin control of gammaherpesvirus latency
Abstract
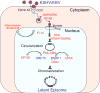
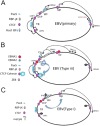
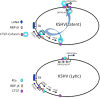
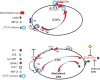
The human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) establish long-term latent infections associated with diverse human cancers. Viral oncogenesis depends on the ability of the latent viral genome to persist in host nuclei as episomes that express a restricted yet dynamic pattern of viral genes. Multiple epigenetic events control viral episome generation and maintenance. This Review highlights some of the recent findings on the role of chromatin assembly, histone and DNA modifications, and higher-order chromosome structures that enable gammaherpesviruses to establish stable latent infections that mediate viral pathogenesis.
Figures

Similar articles
-
Epigenetic regulation of EBV and KSHV latency.Curr Opin Virol. 2013 Jun;3(3):251-9. doi: 10.1016/j.coviro.2013.03.004. Epub 2013 Apr 16. Curr Opin Virol. 2013. PMID: 23601957 Free PMC article. Review.
-
Control of Viral Latency by Episome Maintenance Proteins.Trends Microbiol. 2020 Feb;28(2):150-162. doi: 10.1016/j.tim.2019.09.002. Epub 2019 Oct 14. Trends Microbiol. 2020. PMID: 31624007 Free PMC article. Review.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
Kaposi's sarcoma-associated herpesvirus (KSHV) latency-associated nuclear antigen regulates the KSHV epigenome by association with the histone demethylase KDM3A.J Virol. 2013 Jun;87(12):6782-93. doi: 10.1128/JVI.00011-13. Epub 2013 Apr 10. J Virol. 2013. PMID: 23576503 Free PMC article.
-
An atlas of chromatin landscape in KSHV-infected cells during de novo infection and reactivation.Virology. 2024 Sep;597:110146. doi: 10.1016/j.virol.2024.110146. Epub 2024 Jun 19. Virology. 2024. PMID: 38909515 Review.
Cited by
-
Clinical Manifestations and Epigenetic Regulation of Oral Herpesvirus Infections.Viruses. 2021 Apr 15;13(4):681. doi: 10.3390/v13040681. Viruses. 2021. PMID: 33920978 Free PMC article. Review.
-
Association of Kaposi's Sarcoma-Associated Herpesvirus ORF31 with ORF34 and ORF24 Is Critical for Late Gene Expression.J Virol. 2015 Jun;89(11):6148-54. doi: 10.1128/JVI.00272-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810551 Free PMC article.
-
Navigating the Host Cell Response during Entry into Sites of Latent Cytomegalovirus Infection.Pathogens. 2018 Mar 16;7(1):30. doi: 10.3390/pathogens7010030. Pathogens. 2018. PMID: 29547547 Free PMC article. Review.
-
Epstein-Barr virus (EBV)-encoded small RNAs (EBERs) associated with poor prognosis of head and neck carcinomas.Oncotarget. 2017 Apr 18;8(16):27328-27338. doi: 10.18632/oncotarget.16033. Oncotarget. 2017. PMID: 28423694 Free PMC article.
-
Structural and Functional Basis for an EBNA1 Hexameric Ring in Epstein-Barr Virus Episome Maintenance.J Virol. 2017 Sep 12;91(19):e01046-17. doi: 10.1128/JVI.01046-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701406 Free PMC article.
References
-
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nature reviews Cancer. 2004;4:757–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

