Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis
- PMID: 24174326
- PMCID: PMC4032227
- DOI: 10.1126/scitranslmed.3006869
Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis
Abstract
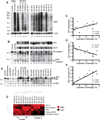
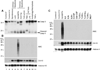
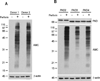
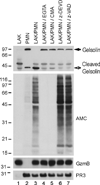
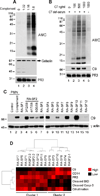
Autoantibodies to citrullinated protein antigens are specific markers of rheumatoid arthritis (RA). Although protein citrullination can be activated by numerous stimuli in cells, it remains unclear which of these produce the prominent citrullinated autoantigens targeted in RA. In these studies, we show that RA synovial fluid cells have an unusual pattern of citrullination with marked citrullination of proteins across the broad range of molecular weights, which we term cellular hypercitrullination. Although histone citrullination is a common event during neutrophil activation and death induced by different pathways including apoptosis, NETosis, and necroptosis/autophagy, hypercitrullination is not induced by these stimuli. However, marked hypercitrullination is induced by two immune-mediated membranolytic pathways, mediated by perforin and the membrane attack complex (MAC), which are active in the RA joint and of importance in RA pathogenesis. We further demonstrate that perforin and MAC activity on neutrophils generate the profile of citrullinated autoantigens characteristic of RA. These data suggest that activation of peptidylarginine deiminases during complement and perforin activity may be at the core of citrullinated autoantigen production in RA. These pathways may be amenable to monitoring and therapeutic modulation.
Figures
Comment in
-
Poking holes in rheumatoid joints.Sci Transl Med. 2013 Oct 30;5(209):209fs39. doi: 10.1126/scitranslmed.3007515. Sci Transl Med. 2013. PMID: 24174325
-
Rheumatoid arthritis: Understanding hypercitrullination in rheumatoid arthritis.Nat Rev Rheumatol. 2014 Jan;10(1):3. doi: 10.1038/nrrheum.2013.182. Epub 2013 Nov 19. Nat Rev Rheumatol. 2014. PMID: 24247366 No abstract available.
Similar articles
-
Rheumatoid arthritis and citrullination.Curr Opin Rheumatol. 2018 Jan;30(1):72-78. doi: 10.1097/BOR.0000000000000452. Curr Opin Rheumatol. 2018. PMID: 28937414 Free PMC article. Review.
-
Generation of Distinct Patterns of Rheumatoid Arthritis Autoantigens by Peptidylarginine Deiminase Types 2 and 4 During Perforin-Induced Cell Damage.Arthritis Rheumatol. 2020 Jun;72(6):912-918. doi: 10.1002/art.41196. Epub 2020 Apr 7. Arthritis Rheumatol. 2020. PMID: 31876120 Free PMC article.
-
Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid.Arthritis Rheumatol. 2015 Dec;67(12):3135-45. doi: 10.1002/art.39313. Arthritis Rheumatol. 2015. PMID: 26245941 Free PMC article.
-
NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis.Sci Transl Med. 2013 Mar 27;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. Sci Transl Med. 2013. PMID: 23536012 Free PMC article.
-
Citrullinated Autoantigens: From Diagnostic Markers to Pathogenetic Mechanisms.Clin Rev Allergy Immunol. 2015 Oct;49(2):232-9. doi: 10.1007/s12016-014-8459-2. Clin Rev Allergy Immunol. 2015. PMID: 25355199 Review.
Cited by
-
Cytoplasmic DNA and AIM2 inflammasome in RA: where they come from and where they go?Front Immunol. 2024 Oct 10;15:1343325. doi: 10.3389/fimmu.2024.1343325. eCollection 2024. Front Immunol. 2024. PMID: 39450183 Free PMC article. Review.
-
Characterization of Neutrophil Functional Responses to SARS-CoV-2 Infection in a Translational Feline Model for COVID-19.Int J Mol Sci. 2024 Sep 19;25(18):10054. doi: 10.3390/ijms251810054. Int J Mol Sci. 2024. PMID: 39337543 Free PMC article.
-
The emerging role of neutrophil extracellular traps in the progression of rheumatoid arthritis.Front Immunol. 2024 Aug 16;15:1438272. doi: 10.3389/fimmu.2024.1438272. eCollection 2024. Front Immunol. 2024. PMID: 39221253 Free PMC article. Review.
-
Gamma-delta T-cell large granular lymphocytic leukemia in the setting of rheumatologic diseases.Front Cell Dev Biol. 2024 Aug 1;12:1434676. doi: 10.3389/fcell.2024.1434676. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39161592 Free PMC article.
-
The molecular basis underlying T cell specificity towards citrullinated epitopes presented by HLA-DR4.Nat Commun. 2024 Jul 23;15(1):6201. doi: 10.1038/s41467-024-50511-w. Nat Commun. 2024. PMID: 39043656 Free PMC article.
References
-
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays. 2003;25:1106–1118. - PubMed
-
- Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, Venables PJ. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol. Rev. 2010;233:34–54. - PubMed
-
- Mortier A, Gouwy M, Van DJ, Proost P. Effect of posttranslational processing on the in vitro and in vivo activity of chemokines. Exp. Cell Res. 2011;317:642–654. - PubMed
-
- Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha- and beta-chains of fibrin. J. Immunol. 2001;166:4177–4184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

