The role of APP and BACE1 trafficking in APP processing and amyloid-β generation
- PMID: 24103387
- PMCID: PMC3978418
- DOI: 10.1186/alzrt211
The role of APP and BACE1 trafficking in APP processing and amyloid-β generation
Abstract
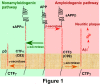
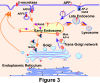
Neuritic plaques in the brain are a major neuropathological hallmark of Alzheimer's disease. They are formed by the deposition and aggregation of extracellular amyloid-β protein (Aβ). Aβ is derived from the sequential cleavage of amyloid-β precursor protein (APP) by β-secretase and γ-secretase. β-Site APP cleaving enzyme 1 (BACE1) functions as the primary, if not sole, β-secretase in vivo and is essential for Aβ production. Regulation of APP processing is a major focus of research into AD pathogenesis. The trafficking systems of APP and its cleavage enzymes are complex. Transporting APP and secretases into the same subcellular organelles facilitates their interaction and favors APP processing. The role of APP and BACE1 trafficking in the amyloidgenic pathway and the underlying mechanisms for Aβ production are discussed in this review. In addition, the distinct mechanisms of amino- and carboxy-terminal Aβ generation are reviewed.
Figures

Similar articles
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
BACE1 Cleavage Site Selection Critical for Amyloidogenesis and Alzheimer's Pathogenesis.J Neurosci. 2017 Jul 19;37(29):6915-6925. doi: 10.1523/JNEUROSCI.0340-17.2017. Epub 2017 Jun 16. J Neurosci. 2017. PMID: 28626014 Free PMC article.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
-
Cellular Trafficking of Amyloid Precursor Protein in Amyloidogenesis Physiological and Pathological Significance.Mol Neurobiol. 2019 Feb;56(2):812-830. doi: 10.1007/s12035-018-1106-9. Epub 2018 May 24. Mol Neurobiol. 2019. PMID: 29797184 Review.
Cited by
-
Amyloidogenesis: What Do We Know So Far?Int J Mol Sci. 2022 Nov 12;23(22):13970. doi: 10.3390/ijms232213970. Int J Mol Sci. 2022. PMID: 36430450 Free PMC article. Review.
-
Altered Protein Palmitoylation as Disease Mechanism in Neurodegenerative Disorders.J Neurosci. 2024 Oct 2;44(40):e1225242024. doi: 10.1523/JNEUROSCI.1225-24.2024. J Neurosci. 2024. PMID: 39358031 Review.
-
Significance of transcytosis in Alzheimer's disease: BACE1 takes the scenic route to axons.Bioessays. 2015 Aug;37(8):888-98. doi: 10.1002/bies.201500019. Epub 2015 Jun 30. Bioessays. 2015. PMID: 26126792 Free PMC article.
-
Amyloid Beta Is Internalized via Macropinocytosis, an HSPG- and Lipid Raft-Dependent and Rac1-Mediated Process.Front Mol Neurosci. 2022 Feb 11;15:804702. doi: 10.3389/fnmol.2022.804702. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36187354 Free PMC article.
-
Dual role of cofilin in APP trafficking and amyloid-β clearance.FASEB J. 2019 Dec;33(12):14234-14247. doi: 10.1096/fj.201901268R. Epub 2019 Oct 24. FASEB J. 2019. PMID: 31646885 Free PMC article.
References
-
- AA-USA. 2011 Alzheimer’s disease facts and figures. W V Med J. 2011;5:82–83. - PubMed
-
- Divry P, Florkin M. Study histochemique senile plaques. J Belge Neurol Psychiatr. 1927;5:643–657.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

