Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function
- PMID: 24091329
- PMCID: PMC3784544
- DOI: 10.1172/JCI69589
Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function
Abstract
Naive CD8+ T cells rely upon oxidation of fatty acids as a primary source of energy. After antigen encounter, T cells shift to a glycolytic metabolism to sustain effector function. It is unclear, however, whether changes in glucose metabolism ultimately influence the ability of activated T cells to become long-lived memory cells. We used a fluorescent glucose analog, 2-NBDG, to quantify glucose uptake in activated CD8+ T cells. We found that cells exhibiting limited glucose incorporation had a molecular profile characteristic of memory precursor cells and an increased capacity to enter the memory pool compared with cells taking up high amounts of glucose. Accordingly, enforcing glycolytic metabolism by overexpressing the glycolytic enzyme phosphoglycerate mutase-1 severely impaired the ability of CD8+ T cells to form long-term memory. Conversely, activation of CD8+ T cells in the presence of an inhibitor of glycolysis, 2-deoxyglucose, enhanced the generation of memory cells and antitumor functionality. Our data indicate that augmenting glycolytic flux drives CD8+ T cells toward a terminally differentiated state, while its inhibition preserves the formation of long-lived memory CD8+ T cells. These results have important implications for improving the efficacy of T cell-based therapies against chronic infectious diseases and cancer.
Figures

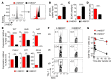

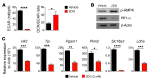


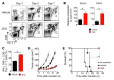
Comment in
-
Tumor cells and memory T cells converge at glycolysis: therapeutic implications.Cancer Biol Ther. 2014 May;15(5):483-5. doi: 10.4161/cbt.28160. Epub 2014 Feb 20. Cancer Biol Ther. 2014. PMID: 24556820 Free PMC article.
Similar articles
-
mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation.J Clin Invest. 2015 May;125(5):2090-108. doi: 10.1172/JCI77746. Epub 2015 Apr 20. J Clin Invest. 2015. PMID: 25893604 Free PMC article.
-
NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity.Nat Immunol. 2021 Feb;22(2):193-204. doi: 10.1038/s41590-020-00829-6. Epub 2021 Jan 4. Nat Immunol. 2021. PMID: 33398181 Free PMC article.
-
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice.Cancer Immunol Immunother. 2016 May;65(5):601-11. doi: 10.1007/s00262-016-1823-8. Epub 2016 Mar 24. Cancer Immunol Immunother. 2016. PMID: 27011014 Free PMC article.
-
mTOR participates in the formation, maintenance, and function of memory CD8+T cells regulated by glycometabolism.Biochem Pharmacol. 2022 Oct;204:115197. doi: 10.1016/j.bcp.2022.115197. Epub 2022 Aug 1. Biochem Pharmacol. 2022. PMID: 35926651 Review.
-
A key control point in the T cell response to chronic infection and neoplasia: FOXO1.Curr Opin Immunol. 2020 Apr;63:51-60. doi: 10.1016/j.coi.2020.02.001. Epub 2020 Mar 2. Curr Opin Immunol. 2020. PMID: 32135399 Free PMC article. Review.
Cited by
-
Lipid and Protein Co-Regulation of PI3K Effectors Akt and Itk in Lymphocytes.Front Immunol. 2015 Mar 13;6:117. doi: 10.3389/fimmu.2015.00117. eCollection 2015. Front Immunol. 2015. PMID: 25821452 Free PMC article. Review.
-
Spermidine Promotes Nb CAR-T Mediated Cytotoxicity to Lymphoma Cells Through Elevating Proliferation and Memory.Onco Targets Ther. 2022 Oct 18;15:1229-1243. doi: 10.2147/OTT.S382540. eCollection 2022. Onco Targets Ther. 2022. PMID: 36267609 Free PMC article.
-
L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity.Cell. 2016 Oct 20;167(3):829-842.e13. doi: 10.1016/j.cell.2016.09.031. Epub 2016 Oct 13. Cell. 2016. PMID: 27745970 Free PMC article.
-
Blocking TCR restimulation induced necroptosis in adoptively transferred T cells improves tumor control.Oncotarget. 2016 Oct 25;7(43):69371-69383. doi: 10.18632/oncotarget.12674. Oncotarget. 2016. PMID: 27750220 Free PMC article.
-
Metabolic regulation of immune responses: therapeutic opportunities.J Clin Invest. 2016 Jun 1;126(6):2031-9. doi: 10.1172/JCI83005. Epub 2016 Jun 1. J Clin Invest. 2016. PMID: 27249676 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

