NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia
- PMID: 24084740
- PMCID: PMC3809785
- DOI: 10.1172/JCI68523
NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia
Abstract
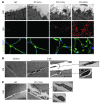
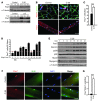
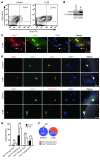
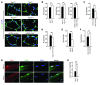
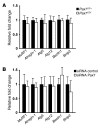
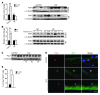
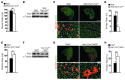
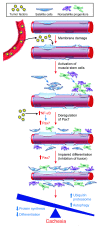
Cachexia is a debilitating condition characterized by extreme skeletal muscle wasting that contributes significantly to morbidity and mortality. Efforts to elucidate the underlying mechanisms of muscle loss have predominantly focused on events intrinsic to the myofiber. In contrast, less regard has been given to potential contributory factors outside the fiber within the muscle microenvironment. In tumor-bearing mice and patients with pancreatic cancer, we found that cachexia was associated with a type of muscle damage resulting in activation of both satellite and nonsatellite muscle progenitor cells. These muscle progenitors committed to a myogenic program, but were inhibited from completing differentiation by an event linked with persistent expression of the self-renewing factor Pax7. Overexpression of Pax7 was sufficient to induce atrophy in normal muscle, while under tumor conditions, the reduction of Pax7 or exogenous addition of its downstream target, MyoD, reversed wasting by restoring cell differentiation and fusion with injured fibers. Furthermore, Pax7 was induced by serum factors from cachectic mice and patients, in an NF-κB-dependent manner, both in vitro and in vivo. Together, these results suggest that Pax7 responds to NF-κB by impairing the regenerative capacity of myogenic cells in the muscle microenvironment to drive muscle wasting in cancer.
Figures


Similar articles
-
Expression of CCAAT/Enhancer Binding Protein Beta in Muscle Satellite Cells Inhibits Myogenesis in Cancer Cachexia.PLoS One. 2015 Dec 28;10(12):e0145583. doi: 10.1371/journal.pone.0145583. eCollection 2015. PLoS One. 2015. PMID: 26709824 Free PMC article.
-
NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia.Science. 2000 Sep 29;289(5488):2363-6. doi: 10.1126/science.289.5488.2363. Science. 2000. PMID: 11009425
-
Long-noncoding RNA Atrolnc-1 promotes muscle wasting in mice with chronic kidney disease.J Cachexia Sarcopenia Muscle. 2018 Oct;9(5):962-974. doi: 10.1002/jcsm.12321. Epub 2018 Jul 24. J Cachexia Sarcopenia Muscle. 2018. PMID: 30043444 Free PMC article.
-
Impaired regeneration: A role for the muscle microenvironment in cancer cachexia.Semin Cell Dev Biol. 2016 Jun;54:82-91. doi: 10.1016/j.semcdb.2015.09.009. Epub 2015 Sep 16. Semin Cell Dev Biol. 2016. PMID: 26385617 Free PMC article. Review.
-
The molecular regulation of muscle stem cell function.Cold Spring Harb Symp Quant Biol. 2008;73:323-31. doi: 10.1101/sqb.2008.73.064. Epub 2009 Mar 27. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19329572 Review.
Cited by
-
Satellite cell activation and apoptosis in skeletal muscle from severely burned children.J Physiol. 2016 Sep 15;594(18):5223-36. doi: 10.1113/JP272520. Epub 2016 Jul 15. J Physiol. 2016. PMID: 27350317 Free PMC article.
-
High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1).J Biol Chem. 2015 Apr 3;290(14):9183-94. doi: 10.1074/jbc.M114.625715. Epub 2015 Feb 17. J Biol Chem. 2015. PMID: 25691571 Free PMC article.
-
Cancer Cachexia: Signaling and Transcriptional Regulation of Muscle Catabolic Genes.Cancers (Basel). 2022 Aug 31;14(17):4258. doi: 10.3390/cancers14174258. Cancers (Basel). 2022. PMID: 36077789 Free PMC article. Review.
-
Extracellular Vesicles and Exosomes in the Control of the Musculoskeletal Health.Curr Osteoporos Rep. 2024 Apr;22(2):257-265. doi: 10.1007/s11914-024-00866-2. Epub 2024 Mar 1. Curr Osteoporos Rep. 2024. PMID: 38424339 Free PMC article. Review.
-
Leukotriene Receptor Antagonist, Montelukast Ameliorates L-NAME-Induced Pre-eclampsia in Rats through Suppressing the IL-6/Jak2/STAT3 Signaling Pathway.Pharmaceuticals (Basel). 2022 Jul 24;15(8):914. doi: 10.3390/ph15080914. Pharmaceuticals (Basel). 2022. PMID: 35893738 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

