Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis
- PMID: 24072267
- PMCID: PMC3953555
- DOI: 10.1007/s11095-013-1212-3
Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis
Abstract
Purpose: To evaluate transcytosis of immunoglobulin G (IgG) by the neonatal Fc receptor (FcRn) in adult primate intestine to determine whether this is a means for oral delivery of monoclonal antibodies (mAbs).
Methods: Relative regional expression of FcRn and localization in human intestinal mucosa by RT-PCR, ELISA & immunohistochemistry. Transcytosis of full-length mAbs (sandwich ELISA-based detection) across human intestinal segments mounted in Ussing-type chambers, human intestinal (caco-2) cell monolayers grown in transwells, and serum levels after regional intestinal delivery in isoflurane-anesthetized cynomolgus monkeys.
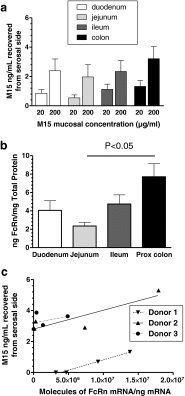
Results: In human intestine, there was an increasing proximal-distal gradient of mucosal FcRn mRNA and protein expression. In cynomolgus, serum mAb levels were greater after ileum-proximal colon infusion than after administration to stomach or proximal small intestine (1-5 mg/kg). Serum levels of wild-type mAb dosed into ileum/proximal colon (2 mg/kg) were 124 ± 104 ng/ml (n = 3) compared to 48 ± 48 ng/ml (n = 2) after a non-FcRn binding variant. In vitro, mAb transcytosis in polarized caco-2 cell monolayers and was not enhanced by increased apical cell surface IgG binding to FcRn. An unexpected finding in primate small intestine, was intense FcRn expression in enteroendocrine cells (chromagranin A, GLP-1 and GLP-2 containing).
Conclusions: In adult primates, FcRn is expressed more highly in distal intestinal epithelial cells. However, mAb delivery to that region results in low serum levels, in part because apical surface FcRn binding does not influence mAb transcytosis. High FcRn expression in enteroendocrine cells could provide a novel means to target mAbs for metabolic diseases after systemic administration.
Figures


Similar articles
-
Contribution of FcRn binding to intestinal uptake of IgG in suckling rat pups and human FcRn-transgenic mice.Am J Physiol Gastrointest Liver Physiol. 2013 Feb 1;304(3):G262-70. doi: 10.1152/ajpgi.00340.2012. Epub 2012 Dec 6. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23220220
-
Impact of mAb-FcRn affinity on IgG transcytosis across human well-differentiated airway epithelium.Front Immunol. 2024 Sep 16;15:1371156. doi: 10.3389/fimmu.2024.1371156. eCollection 2024. Front Immunol. 2024. PMID: 39351230 Free PMC article.
-
Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line.J Clin Invest. 1999 Oct;104(7):903-11. doi: 10.1172/JCI6968. J Clin Invest. 1999. PMID: 10510331 Free PMC article.
-
Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review.Int J Mol Sci. 2022 Aug 24;23(17):9604. doi: 10.3390/ijms23179604. Int J Mol Sci. 2022. PMID: 36077002 Free PMC article. Review.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
Cited by
-
Effect of oregano essential oil on intestinal immunoglobulin G in Holstein dairy bulls.Front Vet Sci. 2024 Jul 29;11:1382396. doi: 10.3389/fvets.2024.1382396. eCollection 2024. Front Vet Sci. 2024. PMID: 39139606 Free PMC article.
-
Passive transfer of COVID-19 immunoglobulin via breast milk post COVID-19 vaccination of lactating mother: case report and review of the literature.Sudan J Paediatr. 2023;23(2):243-247. doi: 10.24911/SJP.106-1664086286. Sudan J Paediatr. 2023. PMID: 38380409 Free PMC article.
-
Exit pathways of therapeutic antibodies from the brain and retention strategies.iScience. 2023 Oct 6;26(11):108132. doi: 10.1016/j.isci.2023.108132. eCollection 2023 Nov 17. iScience. 2023. PMID: 37915602 Free PMC article. Review.
-
Mucus-penetrating and permeation enhancer albumin-based nanoparticles for oral delivery of macromolecules: Application to bevacizumab.Drug Deliv Transl Res. 2024 May;14(5):1189-1205. doi: 10.1007/s13346-023-01454-0. Epub 2023 Oct 26. Drug Deliv Transl Res. 2024. PMID: 37880504 Free PMC article.
-
FcRN receptor antagonists in the management of myasthenia gravis.Front Neurol. 2023 Aug 4;14:1229112. doi: 10.3389/fneur.2023.1229112. eCollection 2023. Front Neurol. 2023. PMID: 37602255 Free PMC article. Review.
References
-
- Jakoi ER, Cambier J, Saslow S. Transepithelial transport of maternal antibody: purification of IgG receptor from newborn rat intestine. J Immunol. 1985;135(5):3360–3364. - PubMed
-
- Berryman M, Rodewald R. Beta 2-microglobulin co-distributes with the heavy chain of the intestinal IgG-Fc receptor throughout the transepithelial transport pathway of the neonatal rat. J Cell Sci. 1995;108(Pt 6):2347–2360. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

