Preclinical development of a novel class of CXCR4 antagonist impairing solid tumors growth and metastases
- PMID: 24058588
- PMCID: PMC3772838
- DOI: 10.1371/journal.pone.0074548
Preclinical development of a novel class of CXCR4 antagonist impairing solid tumors growth and metastases
Abstract
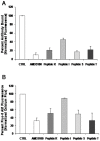
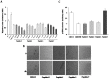
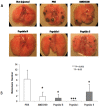
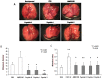
The CXCR4/CXCL12 axis plays a role in cancer metastases, stem cell mobilization and chemosensitization. Proof of concept for efficient CXCR4 inhibition has been demonstrated in stem cell mobilization prior to autologous transplantation in hematological malignancies. Nevertheless CXCR4 inhibitors suitable for prolonged use as required for anticancer therapy are not available. To develop new CXCR4 antagonists a rational, ligand-based approach was taken, distinct from the more commonly used development strategy. A three amino acid motif (Ar-Ar-X) in CXCL12, also found in the reverse orientation (X-Ar-Ar) in the vMIP-II inhibitory chemokine formed the core of nineteen cyclic peptides evaluated for inhibition of CXCR4-dependent migration, binding, P-ERK1/2-induction and calcium efflux. Peptides R, S and I were chosen for evaluation in in vivo models of lung metastases (B16-CXCR4 and KTM2 murine osteosarcoma cells) and growth of a renal cells xenograft. Peptides R, S, and T significantly reduced the association of the 12G5-CXCR4 antibody to the receptor and inhibited CXCL12-induced calcium efflux. The four peptides efficiently inhibited CXCL12-dependent migration at concentrations as low as 10 nM and delayed CXCL12-mediated wound healing in PES43 human melanoma cells. Intraperitoneal treatment with peptides R, I or S drastically reduced the number of B16-CXCR4-derived lung metastases in C57/BL mice. KTM2 osteosarcoma lung metastases were also reduced in Balb/C mice following CXCR4 inhibition. All three peptides significantly inhibited subcutaneous growth of SN12C-EGFP renal cancer cells. A novel class of CXCR4 inhibitory peptides was discovered. Three peptides, R, I and S inhibited lung metastases and primary tumor growth and will be evaluated as anticancer agents.
Conflict of interest statement
Figures
Similar articles
-
Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases.Clin Exp Metastasis. 2008;25(3):201-11. doi: 10.1007/s10585-007-9133-3. Epub 2007 Dec 11. Clin Exp Metastasis. 2008. PMID: 18071913 Free PMC article.
-
Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases.Mol Cancer Ther. 2006 Oct;5(10):2592-9. doi: 10.1158/1535-7163.MCT-06-0310. Mol Cancer Ther. 2006. PMID: 17041104 Free PMC article.
-
Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models.Mol Cancer Ther. 2015 Feb;14(2):480-90. doi: 10.1158/1535-7163.MCT-14-0850. Epub 2014 Dec 12. Mol Cancer Ther. 2015. PMID: 25504752
-
Dissecting the role of the CXCL12/CXCR4 axis in acute myeloid leukaemia.Br J Haematol. 2020 Jun;189(5):815-825. doi: 10.1111/bjh.16456. Epub 2020 Mar 5. Br J Haematol. 2020. PMID: 32135579 Review.
-
The chemokine receptors CXCR4/CXCR7 and their primary heterodimeric ligands CXCL12 and CXCL12/high mobility group box 1 in pancreatic cancer growth and development: finding flow.Pancreas. 2015 May;44(4):528-34. doi: 10.1097/MPA.0000000000000298. Pancreas. 2015. PMID: 25872129 Review.
Cited by
-
Conformational ensembles explored dynamically from disordered peptides targeting chemokine receptor CXCR4.Int J Mol Sci. 2015 May 28;16(6):12159-73. doi: 10.3390/ijms160612159. Int J Mol Sci. 2015. PMID: 26030674 Free PMC article.
-
New Insights on the Emerging Genomic Landscape of CXCR4 in Cancer: A Lesson from WHIM.Vaccines (Basel). 2020 Apr 3;8(2):164. doi: 10.3390/vaccines8020164. Vaccines (Basel). 2020. PMID: 32260318 Free PMC article. Review.
-
Cotargeting of miR-126-3p and miR-221-3p inhibits PIK3R2 and PTEN, reducing lung cancer growth and metastasis by blocking AKT and CXCR4 signalling.Mol Oncol. 2021 Nov;15(11):2969-2988. doi: 10.1002/1878-0261.13036. Epub 2021 Jul 21. Mol Oncol. 2021. PMID: 34107168 Free PMC article.
-
CXCL12 Signaling in the Tumor Microenvironment.Adv Exp Med Biol. 2021;1302:51-70. doi: 10.1007/978-3-030-62658-7_5. Adv Exp Med Biol. 2021. PMID: 34286441 Review.
-
At the Bench: Pre-clinical evidence for multiple functions of CXCR4 in cancer.J Leukoc Biol. 2021 May;109(5):969-989. doi: 10.1002/JLB.2BT1018-715RR. Epub 2020 Oct 26. J Leukoc Biol. 2021. PMID: 33104270 Free PMC article. Review.
References
-
- Baggiolini M (2001) Chemokines in pathology and medicine. J Intern Med 250: 91–104. - PubMed
-
- Allen SJ, Crown SE, Handel TM (2007) Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 25: 787–820. - PubMed
-
- Wu X, Lee VC, Chevalier E, Hwang ST (2009) Chemokine receptors as targets for cancer therapy. Curr Pharm Des 2009 15: 742–57. - PubMed
-
- Teicher BA, Fricker SP (2010) CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 16: 2927–31. - PubMed
-
- Muller A, Homey B, Soto H, Ge N, Catron D, et al. (2001) Involvement of chemokine receptors in breast cancer metastasis. Nature 410: 50–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

