Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies
- PMID: 23996115
- PMCID: PMC3836053
- DOI: 10.1007/s10928-013-9332-2
Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies
Abstract
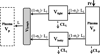
Minimal physiologically-based pharmacokinetic (mPBPK) models provide a sensible modeling approach when fitting only plasma (or blood) data yielding physiologically-relevant PK parameters that may provide more practical value than parameters of mammillary models. We propose a second-generation mPBPK model specifically for monoclonal antibodies (mAb) by including (lumping) several essential components of mAb PK used in full PBPK models. These components include convection as the primary mechanism of antibody movement from plasma into tissues and return to plasma with interstitial fluid as the major extravascular distribution space. The model divides tissue spaces into two groups according to their vascular endothelial structure, leaky and tight, which consequently allows discernment of two types and general sites of distribution. This mPBPK model was applied to two mAbs in mice and ten mAbs with linear kinetics in humans. The model captured their plasma PK profiles well with predictions of concentrations in interstitial fluid for two types of tissues. Predictions of tissue concentrations for mAb 7E3 and 8C2 were consistent with actual measurements in mice, indicating the feasibility of this model in assessing extravascular distribution in the two categories of tissues. The vascular reflection coefficients (σ₁) of tight tissues (V(tight)) ranged 0.883-0.987 and coefficients (σ₂) for leaky tissues (V(leaky)) ranged 0.311 to 0.837. The plasma clearance (CL(p)) varied among the mAbs in humans from 0.0054 to 0.03 L/h. In addition, applying this model generates parameters for mAb transcapillary escape rates and assesses major sites of elimination. Four of ten mAbs exhibited better fitting statistics premised on elimination from interstitial fluid than from plasma. This approach allows comparisons of mAb PK when only plasma data are available, provides more realistic parameters and predictions than mammillary models, and may provide an intermediate step towards utilizing full PBPK models for mAbs.
Figures




Similar articles
-
Survey of monoclonal antibody disposition in man utilizing a minimal physiologically-based pharmacokinetic model.J Pharmacokinet Pharmacodyn. 2014 Dec;41(6):571-80. doi: 10.1007/s10928-014-9374-0. Epub 2014 Aug 22. J Pharmacokinet Pharmacodyn. 2014. PMID: 25146360 Free PMC article.
-
Across-Species Scaling of Monoclonal Antibody Pharmacokinetics Using a Minimal PBPK Model.Pharm Res. 2015 Oct;32(10):3269-81. doi: 10.1007/s11095-015-1703-5. Epub 2015 May 5. Pharm Res. 2015. PMID: 25939552 Free PMC article.
-
Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human.J Pharmacokinet Pharmacodyn. 2012 Feb;39(1):67-86. doi: 10.1007/s10928-011-9232-2. Epub 2011 Dec 6. J Pharmacokinet Pharmacodyn. 2012. PMID: 22143261
-
Review of the Existing Translational Pharmacokinetics Modeling Approaches Specific to Monoclonal Antibodies (mAbs) to Support the First-In-Human (FIH) Dose Selection.Int J Mol Sci. 2022 Oct 22;23(21):12754. doi: 10.3390/ijms232112754. Int J Mol Sci. 2022. PMID: 36361546 Free PMC article. Review.
-
Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development.Drug Metab Pharmacokinet. 2019 Feb;34(1):3-13. doi: 10.1016/j.dmpk.2018.11.002. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30522890 Free PMC article. Review.
Cited by
-
A Meta-Analysis Methodology in Stan to Estimate Population Pharmacokinetic Parameters from Multiple Aggregate Concentration-Time Datasets: Application to Gevokizumab mPBPK Model.Pharmaceutics. 2024 Aug 27;16(9):1129. doi: 10.3390/pharmaceutics16091129. Pharmaceutics. 2024. PMID: 39339167 Free PMC article.
-
Immunoglobulin therapies for primary immunodeficiency diseases (part 1): understanding the pharmacokinetics.Immunotherapy. 2024;16(13):879-894. doi: 10.1080/1750743X.2024.2382081. Epub 2024 Sep 26. Immunotherapy. 2024. PMID: 39323402 Free PMC article. Review.
-
ISB 2001 trispecific T cell engager shows strong tumor cytotoxicity and overcomes immune escape mechanisms of multiple myeloma cells.Nat Cancer. 2024 Oct;5(10):1494-1514. doi: 10.1038/s43018-024-00821-1. Epub 2024 Sep 11. Nat Cancer. 2024. PMID: 39261676 Free PMC article.
-
A Minimal PBPK/PD Model with Expansion-Enhanced Target-Mediated Drug Disposition to Support a First-in-Human Clinical Study Design for a FLT3L-Fc Molecule.Pharmaceutics. 2024 May 15;16(5):660. doi: 10.3390/pharmaceutics16050660. Pharmaceutics. 2024. PMID: 38794321 Free PMC article.
-
A minimal physiologically based pharmacokinetic model to study the combined effect of antibody size, charge, and binding affinity to FcRn/antigen on antibody pharmacokinetics.J Pharmacokinet Pharmacodyn. 2024 Feb 24. doi: 10.1007/s10928-023-09899-z. Online ahead of print. J Pharmacokinet Pharmacodyn. 2024. PMID: 38400996
References
-
- Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. - PubMed
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9:767–774. - PubMed
-
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. - PubMed
-
- Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24:23–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

