Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice
- PMID: 23951048
- PMCID: PMC3739772
- DOI: 10.1371/journal.pone.0070945
Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice
Abstract
Introduction: Alcohol-induced neuroinflammation is mediated by pro-inflammatory cytokines and chemokines including tumor necrosis factor-α (TNFα), monocyte chemotactic protein-1 (MCP1) and interleukin-1-beta (IL-1β). Toll-like receptor-4 (TLR4) pathway induced nuclear factor-κB (NF-κB) activation is involved in the pathogenesis of alcohol-induced neuroinflammation. Inflammation is a highly regulated process. Recent studies suggest that microRNAs (miRNAs) play crucial role in fine tuning gene expression and miR-155 is a major regulator of inflammation in immune cells after TLR stimulation.
Aim: To evaluate the role of miR-155 in the pathogenesis of alcohol-induced neuroinflammation.
Methods: Wild type (WT), miR-155- and TLR4-knockout (KO) mice received 5% ethanol-containing or isocaloric control diet for 5 weeks. Microglia markers were measured by q-RTPCR; inflammasome activation was measured by enzyme activity; TNFα, MCP1, IL-1β mRNA and protein were measured by q-RTPCR and ELISA; phospho-p65 protein and NF-κB were measured by Western-blotting and EMSA; miRNAs were measured by q-PCR in the cerebellum. MiR-155 was measured in immortalized and primary mouse microglia after lipopolysaccharide and ethanol stimulation.
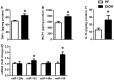
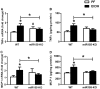
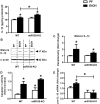
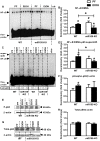
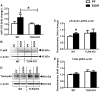
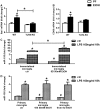
Results: Chronic ethanol feeding up-regulated miR-155 and miR-132 expression in mouse cerebellum. Deficiency in miR-155 protected mice from alcohol-induced increase in inflammatory cytokines; TNFα, MCP1 protein and TNFα, MCP1, pro-IL-1β and pro-caspase-1 mRNA levels were reduced in miR-155 KO alcohol-fed mice. NF-κB was activated in WT but not in miR-155 KO alcohol-fed mice. However increases in cerebellar caspase-1 activity and IL-1β levels were similar in alcohol-fed miR-155-KO and WT mice. Alcohol-fed TLR4-KO mice were protected from the induction of miR-155. NF-κB activation measured by phosphorylation of p65 and neuroinflammation were reduced in alcohol-fed TLR4-KO compared to control mice. TLR4 stimulation with lipopolysaccharide in primary or immortalized mouse microglia resulted in increased miR-155.
Conclusion: Chronic alcohol induces miR-155 in the cerebellum in a TLR4-dependent manner. Alcohol-induced miR-155 regulates TNFα and MCP1 expression but not caspase-dependent IL-1β increase in neuroinflammation.
Conflict of interest statement
Figures
Similar articles
-
Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation.J Leukoc Biol. 2013 Jul;94(1):171-82. doi: 10.1189/jlb.1212659. Epub 2013 Apr 26. J Leukoc Biol. 2013. PMID: 23625200 Free PMC article.
-
MicroRNA-155 deficiency attenuates inflammation and oxidative stress in experimental autoimmune prostatitis in a TLR4-dependent manner.Kaohsiung J Med Sci. 2020 Sep;36(9):712-720. doi: 10.1002/kjm2.12229. Epub 2020 May 21. Kaohsiung J Med Sci. 2020. PMID: 32436368
-
Two-Month Voluntary Ethanol Consumption Promotes Mild Neuroinflammation in the Cerebellum but Not in the Prefrontal Cortex, Hippocampus, or Striatum of Mice.Int J Mol Sci. 2024 Apr 10;25(8):4173. doi: 10.3390/ijms25084173. Int J Mol Sci. 2024. PMID: 38673763 Free PMC article.
-
Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway.J Neuroinflammation. 2019 Jul 18;16(1):148. doi: 10.1186/s12974-019-1538-9. J Neuroinflammation. 2019. PMID: 31319868 Free PMC article. Review.
-
Converging actions of alcohol on liver and brain immune signaling.Int Rev Neurobiol. 2014;118:359-80. doi: 10.1016/B978-0-12-801284-0.00011-7. Int Rev Neurobiol. 2014. PMID: 25175869 Review.
Cited by
-
Detrimental Effects of Alcohol-Induced Inflammation on Brain Health: From Neurogenesis to Neurodegeneration.Cell Mol Neurobiol. 2023 Jul;43(5):1885-1904. doi: 10.1007/s10571-022-01308-2. Epub 2022 Nov 27. Cell Mol Neurobiol. 2023. PMID: 36436159 Review.
-
MicroRNAs in alcoholic liver disease.Semin Liver Dis. 2015 Feb;35(1):36-42. doi: 10.1055/s-0034-1397347. Epub 2015 Jan 29. Semin Liver Dis. 2015. PMID: 25632933 Free PMC article. Review.
-
Down-regulation of ROCK2 alleviates ethanol-induced cerebral nerve injury partly by the suppression of the NF-κB signaling pathway.Bioengineered. 2020 Dec;11(1):779-790. doi: 10.1080/21655979.2020.1795404. Bioengineered. 2020. PMID: 32684089 Free PMC article.
-
Oleoylethanolamide, Neuroinflammation, and Alcohol Abuse.Front Mol Neurosci. 2019 Jan 9;11:490. doi: 10.3389/fnmol.2018.00490. eCollection 2018. Front Mol Neurosci. 2019. PMID: 30687006 Free PMC article. Review.
-
Midkine in the mouse ventral tegmental area limits ethanol intake and Ccl2 gene expression.Genes Brain Behav. 2017 Sep;16(7):699-708. doi: 10.1111/gbb.12384. Epub 2017 May 2. Genes Brain Behav. 2017. PMID: 28398003 Free PMC article.
References
-
- World Health Organization website. Available: Http://Www.who.int/mediacentre/factsheets/fs349/en/index.html. Accessed 2011 Feb.
-
- Thounaojam MC, Kaushik DK, Basu A (2013) MicroRNAs in the brain: It’s regulatory role in neuroinflammation. Mol Neurobiol 47: 1034–1044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

