Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood
- PMID: 23897980
- PMCID: PMC3754857
- DOI: 10.1084/jem.20130592
Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood
Abstract
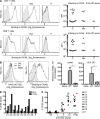
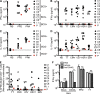
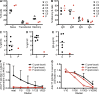
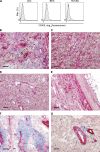
Kaposi sarcoma (KS), a human herpes virus 8 (HHV-8; also called KSHV)-induced endothelial tumor, develops only in a small fraction of individuals infected with HHV-8. We hypothesized that inborn errors of immunity to HHV-8 might underlie the exceedingly rare development of classic KS in childhood. We report here autosomal recessive OX40 deficiency in an otherwise healthy adult with childhood-onset classic KS. OX40 is a co-stimulatory receptor expressed on activated T cells. Its ligand, OX40L, is expressed on various cell types, including endothelial cells. We found OX40L was abundantly expressed in KS lesions. The mutant OX40 protein was poorly expressed on the cell surface and failed to bind OX40L, resulting in complete functional OX40 deficiency. The patient had a low proportion of effector memory CD4(+) T cells in the peripheral blood, consistent with impaired CD4(+) T cell responses to recall antigens in vitro. The proportion of effector memory CD8(+) T cells was less diminished. The proportion of circulating memory B cells was low, but the antibody response in vivo was intact, including the response to a vaccine boost. Together, these findings suggest that human OX40 is necessary for robust CD4(+) T cell memory and confers apparently selective protective immunity against HHV-8 infection in endothelial cells.
Figures




Similar articles
-
Kaposi Sarcoma of Childhood: Inborn or Acquired Immunodeficiency to Oncogenic HHV-8.Pediatr Blood Cancer. 2016 Mar;63(3):392-7. doi: 10.1002/pbc.25779. Epub 2015 Oct 15. Pediatr Blood Cancer. 2016. PMID: 26469702 Free PMC article. Review.
-
OX40 and 4-1BB downregulate Kaposi’s sarcoma-associated herpesvirus replication in lymphatic endothelial cells, but 4-1BB and not OX40 inhibits viral replication in B-cells.J Gen Virol. 2015 Dec;96(12):3635-3645. doi: 10.1099/jgv.0.000312. J Gen Virol. 2015. PMID: 26467721
-
Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma.J Exp Med. 2010 Oct 25;207(11):2307-12. doi: 10.1084/jem.20101597. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876309 Free PMC article.
-
Requirements for the functional expression of OX40 ligand on human activated CD4+ and CD8+ T cells.Hum Immunol. 2007 Jul;68(7):563-71. doi: 10.1016/j.humimm.2007.03.012. Epub 2007 Apr 13. Hum Immunol. 2007. PMID: 17584577
-
OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology.Adv Immunol. 2010;105:63-98. doi: 10.1016/S0065-2776(10)05003-0. Adv Immunol. 2010. PMID: 20510730 Review.
Cited by
-
Next-Generation Sequencing in the Understanding of Kaposi's Sarcoma-Associated Herpesvirus (KSHV) Biology.Viruses. 2016 Mar 31;8(4):92. doi: 10.3390/v8040092. Viruses. 2016. PMID: 27043613 Free PMC article. Review.
-
The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses.Immunity. 2016 May 17;44(5):1005-19. doi: 10.1016/j.immuni.2016.04.019. Immunity. 2016. PMID: 27192566 Free PMC article. Review.
-
The Spectrum of Underlying Causes of Iatrogenic Kaposi's Sarcoma in a Large Series: A Retrospective Study.Indian J Dermatol. 2019 Sep-Oct;64(5):392-399. doi: 10.4103/ijd.IJD_217_18. Indian J Dermatol. 2019. PMID: 31543535 Free PMC article.
-
Primary lymphocyte infection models for KSHV and its putative tumorigenesis mechanisms in B cell lymphomas.J Microbiol. 2017 May;55(5):319-329. doi: 10.1007/s12275-017-7075-2. Epub 2017 Apr 29. J Microbiol. 2017. PMID: 28455586 Review.
-
OX40 Cooperates with ICOS To Amplify Follicular Th Cell Development and Germinal Center Reactions during Infection.J Immunol. 2017 Jan 1;198(1):218-228. doi: 10.4049/jimmunol.1601356. Epub 2016 Nov 28. J Immunol. 2017. PMID: 27895177 Free PMC article.
References
-
- Akman E.S., Ertem U., Tankal V., Pamir A., Tuncer A.M., Uluoğlu O. 1989. Aggressive Kaposi’s sarcoma in children: a case report. Turk. J. Pediatr. 31:297–303 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

