Phosphorylation regulates FOXC2-mediated transcription in lymphatic endothelial cells
- PMID: 23878394
- PMCID: PMC3811871
- DOI: 10.1128/MCB.01387-12
Phosphorylation regulates FOXC2-mediated transcription in lymphatic endothelial cells
Abstract
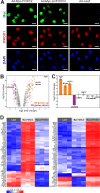
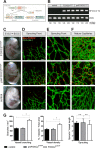
One of the key mechanisms linking cell signaling and control of gene expression is reversible phosphorylation of transcription factors. FOXC2 is a forkhead transcription factor that is mutated in the human vascular disease lymphedema-distichiasis and plays an essential role in lymphatic vascular development. However, the mechanisms regulating FOXC2 transcriptional activity are not well understood. We report here that FOXC2 is phosphorylated on eight evolutionarily conserved proline-directed serine/threonine residues. Loss of phosphorylation at these sites triggers substantial changes in the FOXC2 transcriptional program. Through genome-wide location analysis in lymphatic endothelial cells, we demonstrate that the changes are due to selective inhibition of FOXC2 recruitment to chromatin. The extent of the inhibition varied between individual binding sites, suggesting a novel rheostat-like mechanism by which expression of specific genes can be differentially regulated by FOXC2 phosphorylation. Furthermore, unlike the wild-type protein, the phosphorylation-deficient mutant of FOXC2 failed to induce vascular remodeling in vivo. Collectively, our results point to the pivotal role of phosphorylation in the regulation of FOXC2-mediated transcription in lymphatic endothelial cells and underscore the importance of FOXC2 phosphorylation in vascular development.
Figures



Similar articles
-
Endothelial immune activation programmes cell-fate decisions and angiogenesis by inducing angiogenesis regulator DLL4 through TLR4-ERK-FOXC2 signalling.J Physiol. 2018 Apr 15;596(8):1397-1417. doi: 10.1113/JP275453. Epub 2018 Mar 2. J Physiol. 2018. PMID: 29380370 Free PMC article.
-
Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation.Elife. 2020 Jun 8;9:e53814. doi: 10.7554/eLife.53814. Elife. 2020. PMID: 32510325 Free PMC article.
-
Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2.Nat Commun. 2015 Jun 1;6:7274. doi: 10.1038/ncomms8274. Nat Commun. 2015. PMID: 26027726
-
FOXC2 transcription factor: a novel regulator of lymphangiogenesis.Lymphology. 2011 Mar;44(1):35-41. Lymphology. 2011. PMID: 21667821 Review.
-
Novel FOXC2 missense mutation identified in patient with lymphedema-distichiasis syndrome and review.Lymphology. 2008 Sep;41(3):98-102. Lymphology. 2008. PMID: 19013876 Review.
Cited by
-
FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature.J Clin Invest. 2015 Oct 1;125(10):3861-77. doi: 10.1172/JCI80454. Epub 2015 Sep 21. J Clin Invest. 2015. PMID: 26389677 Free PMC article.
-
Imbalance between Expression of FOXC2 and Its lncRNA in Lymphedema-Distichiasis Caused by Frameshift Mutations.Genes (Basel). 2021 Apr 27;12(5):650. doi: 10.3390/genes12050650. Genes (Basel). 2021. PMID: 33925370 Free PMC article.
-
FOXC2 regulates the G2/M transition of stem cell-rich breast cancer cells and sensitizes them to PLK1 inhibition.Sci Rep. 2016 Apr 11;6:23070. doi: 10.1038/srep23070. Sci Rep. 2016. PMID: 27064522 Free PMC article.
-
FOXC2 Autoregulates Its Expression in the Pulmonary Endothelium After Endotoxin Stimulation in a Histone Acetylation-Dependent Manner.Front Cell Dev Biol. 2021 May 4;9:657662. doi: 10.3389/fcell.2021.657662. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34017833 Free PMC article.
-
Distinct transcriptional responses of lymphatic endothelial cells to VEGFR-3 and VEGFR-2 stimulation.Sci Data. 2017 Aug 29;4:170106. doi: 10.1038/sdata.2017.106. Sci Data. 2017. PMID: 28850122 Free PMC article.
References
-
- Iida K, Koseki H, Kakinuma H, Kato N, Mizutani-Koseki Y, Ohuchi H, Yoshioka H, Noji S, Kawamura K, Kataoka Y, Ueno F, Taniguchi M, Yoshida N, Sugiyama T, Miura N. 1997. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development 124:4627–4638 - PubMed
-
- Winnier GE, Kume T, Deng K, Rogers R, Bundy J, Raines C, Walter MA, Hogan BL, Conway SJ. 1999. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev. Biol. 213:418–431 - PubMed
-
- Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. 2004. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 10:974–981 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
