Molecular basis of glycosaminoglycan heparin binding to the chemokine CXCL1 dimer
- PMID: 23864653
- PMCID: PMC3757178
- DOI: 10.1074/jbc.M113.492579
Molecular basis of glycosaminoglycan heparin binding to the chemokine CXCL1 dimer
Abstract
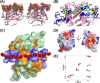
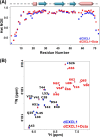
Glycosaminoglycan (GAG)-bound and soluble chemokine gradients in the vasculature and extracellular matrix mediate neutrophil recruitment to the site of microbial infection and sterile injury in the host tissue. However, the molecular principles by which chemokine-GAG interactions orchestrate these gradients are poorly understood. This, in part, can be directly attributed to the complex interrelationship between the chemokine monomer-dimer equilibrium and binding geometry and affinities that are also intimately linked to GAG length. To address some of this missing knowledge, we have characterized the structural basis of heparin binding to the murine CXCL1 dimer. CXCL1 is a neutrophil-activating chemokine and exists as both monomers and dimers (Kd = 36 μm). To avoid interference from monomer-GAG interactions, we designed a trapped dimer (dCXCL1) by introducing a disulfide bridge across the dimer interface. We characterized the binding of GAG heparin octasaccharide to dCXCL1 using solution NMR spectroscopy. Our studies show that octasaccharide binds orthogonally to the interhelical axis and spans the dimer interface and that heparin binding enhances the structural integrity of the C-terminal helical residues and stability of the dimer. We generated a quadruple mutant (H20A/K22A/K62A/K66A) on the basis of the binding data and observed that this mutant failed to bind heparin octasaccharide, validating our structural model. We propose that the stability enhancement of dimers upon GAG binding regulates in vivo neutrophil trafficking by increasing the lifetime of "active" chemokines, and that this structural knowledge could be exploited for designing inhibitors that disrupt chemokine-GAG interactions and neutrophil homing to the target tissue.
Keywords: Chemokines; Glycobiology; Glycosaminoglycan; Heparin; NMR; Neutrophil.
Figures


Similar articles
-
Structural basis, stoichiometry, and thermodynamics of binding of the chemokines KC and MIP2 to the glycosaminoglycan heparin.J Biol Chem. 2018 Nov 16;293(46):17817-17828. doi: 10.1074/jbc.RA118.004866. Epub 2018 Sep 26. J Biol Chem. 2018. PMID: 30257866 Free PMC article.
-
Molecular Basis of Chemokine CXCL5-Glycosaminoglycan Interactions.J Biol Chem. 2016 Sep 23;291(39):20539-50. doi: 10.1074/jbc.M116.745265. Epub 2016 Jul 28. J Biol Chem. 2016. PMID: 27471273 Free PMC article.
-
CXCL1/MGSA Is a Novel Glycosaminoglycan (GAG)-binding Chemokine: STRUCTURAL EVIDENCE FOR TWO DISTINCT NON-OVERLAPPING BINDING DOMAINS.J Biol Chem. 2016 Feb 19;291(8):4247-55. doi: 10.1074/jbc.M115.697888. Epub 2015 Dec 31. J Biol Chem. 2016. PMID: 26721883 Free PMC article.
-
Glycosaminoglycan Interactions Fine-Tune Chemokine-Mediated Neutrophil Trafficking: Structural Insights and Molecular Mechanisms.J Histochem Cytochem. 2018 Apr;66(4):229-239. doi: 10.1369/0022155417739864. Epub 2018 Jan 1. J Histochem Cytochem. 2018. PMID: 29290145 Free PMC article. Review.
-
Structural Insights Into How Proteoglycans Determine Chemokine-CXCR1/CXCR2 Interactions: Progress and Challenges.Front Immunol. 2020 Apr 24;11:660. doi: 10.3389/fimmu.2020.00660. eCollection 2020. Front Immunol. 2020. PMID: 32391006 Free PMC article. Review.
Cited by
-
Structural basis, stoichiometry, and thermodynamics of binding of the chemokines KC and MIP2 to the glycosaminoglycan heparin.J Biol Chem. 2018 Nov 16;293(46):17817-17828. doi: 10.1074/jbc.RA118.004866. Epub 2018 Sep 26. J Biol Chem. 2018. PMID: 30257866 Free PMC article.
-
Interleukin-37 monomer is the active form for reducing innate immunity.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5514-5522. doi: 10.1073/pnas.1819672116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819901 Free PMC article.
-
Solution structure of CXCL5--a novel chemokine and adipokine implicated in inflammation and obesity.PLoS One. 2014 Apr 2;9(4):e93228. doi: 10.1371/journal.pone.0093228. eCollection 2014. PLoS One. 2014. PMID: 24695525 Free PMC article.
-
Mechanistic and therapeutic overview of glycosaminoglycans: the unsung heroes of biomolecular signaling.Glycoconj J. 2016 Feb;33(1):1-17. doi: 10.1007/s10719-015-9642-2. Epub 2015 Dec 3. Glycoconj J. 2016. PMID: 26635091 Review.
-
Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins.BMC Cancer. 2014 Oct 24;14:781. doi: 10.1186/1471-2407-14-781. BMC Cancer. 2014. PMID: 25344051 Free PMC article.
References
-
- Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 - PubMed
-
- Wang L., Fuster M., Sriramarao P., Esko J. D. (2005) Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 - PubMed
-
- Parish C. R. (2006) The role of heparan sulphate in inflammation. Nat. Rev. Immunol. 6, 633–643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

