Oxidant sensing by reversible disulfide bond formation
- PMID: 23861395
- PMCID: PMC3772196
- DOI: 10.1074/jbc.R113.462929
Oxidant sensing by reversible disulfide bond formation
Abstract
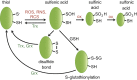
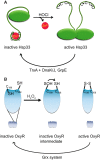
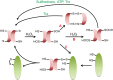
Maintenance of the cellular redox balance is crucial for cell survival. An increase in reactive oxygen, nitrogen, or chlorine species can lead to oxidative stress conditions, potentially damaging DNA, lipids, and proteins. Proteins are very sensitive to oxidative modifications, particularly methionine and cysteine residues. The reversibility of some of these oxidative protein modifications makes them ideally suited to take on regulatory roles in protein function. This is especially true for disulfide bond formation, which has the potential to mediate extensive yet fully reversible structural and functional changes, rapidly adjusting the protein's activity to the prevailing oxidant levels.
Keywords: Antioxidants; Oxidative Stress; Reactive Oxygen Species (ROS); Redox Signaling; Stress Response.
Figures
Similar articles
-
Oxidation of biological thiols by highly reactive disulfide-S-oxides.Gen Physiol Biophys. 2002 Mar;21(1):65-72. Gen Physiol Biophys. 2002. PMID: 12168727
-
Trapping redox partnerships in oxidant-sensitive proteins with a small, thiol-reactive cross-linker.Free Radic Biol Med. 2016 Dec;101:356-366. doi: 10.1016/j.freeradbiomed.2016.10.506. Epub 2016 Nov 2. Free Radic Biol Med. 2016. PMID: 27816612 Free PMC article.
-
Global methods to monitor the thiol-disulfide state of proteins in vivo.Antioxid Redox Signal. 2006 May-Jun;8(5-6):763-72. doi: 10.1089/ars.2006.8.763. Antioxid Redox Signal. 2006. PMID: 16771668 Review.
-
Protein redox modification as a cellular defense mechanism against tissue ischemic injury.Oxid Med Cell Longev. 2014;2014:343154. doi: 10.1155/2014/343154. Epub 2014 May 5. Oxid Med Cell Longev. 2014. PMID: 24883175 Free PMC article. Review.
-
Oxidoreduction of protein thiols in redox regulation.Biochem Soc Trans. 2005 Dec;33(Pt 6):1378-81. doi: 10.1042/BST0331378. Biochem Soc Trans. 2005. PMID: 16246123 Review.
Cited by
-
Glutamine Metabolism Regulates the Pluripotency Transcription Factor OCT4.Cell Rep. 2016 Jul 12;16(2):323-332. doi: 10.1016/j.celrep.2016.05.089. Epub 2016 Jun 23. Cell Rep. 2016. PMID: 27346346 Free PMC article.
-
Protein S-sulfenylation is a fleeting molecular switch that regulates non-enzymatic oxidative folding.Nat Commun. 2016 Aug 22;7:12490. doi: 10.1038/ncomms12490. Nat Commun. 2016. PMID: 27546612 Free PMC article.
-
Validation of the Intermolecular Disulfide Bond in Caspase-2.Biology (Basel). 2024 Jan 17;13(1):49. doi: 10.3390/biology13010049. Biology (Basel). 2024. PMID: 38248479 Free PMC article.
-
Preliminary results of clinical, biochemical, and radiological investigation into the oxidative status in patients with rotator cuff tendinopathy.Acta Orthop Traumatol Turc. 2024 Jun 27;58(3):161-166. doi: 10.5152/j.aott.2024.22030. Acta Orthop Traumatol Turc. 2024. PMID: 39165100 Free PMC article.
-
Antibacterial strategies inspired by the oxidative stress and response networks.J Microbiol. 2019 Mar;57(3):203-212. doi: 10.1007/s12275-019-8711-9. Epub 2019 Feb 26. J Microbiol. 2019. PMID: 30806977 Review.
References
-
- Alam M. S., Garg S. K., Agrawal P. (2009) Studies on structural and functional divergence among seven WhiB proteins of Mycobacterium tuberculosis H37Rv. FEBS J. 276, 76–93 - PubMed
-
- Holmgren A., Johansson C., Berndt C., Lönn M. E., Hudemann C., Lillig C. H. (2005) Thiol redox control via thioredoxin and glutaredoxin systems. Biochem. Soc. Trans. 33, 1375–1377 - PubMed
-
- Jakob U., Eser M., Bardwell J. C. A. (2000) Redox switch of Hsp33 has a novel zinc-binding motif. J. Biol. Chem. 275, 38302–38310 - PubMed
-
- Lee C., Lee S. M., Mukhopadhyay P., Kim S. J., Lee S. C., Ahn W. S., Yu M. H., Storz G., Ryu S. E. (2004) Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat. Struct. Mol. Biol. 11, 1179–1185 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

