Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds
- PMID: 23811217
- PMCID: PMC3791182
- DOI: 10.1016/j.actbio.2013.06.021
Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds
Erratum in
-
Corrigendum to "Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds" [Acta Biomater. 9(11) (2013) 8802-8814].Acta Biomater. 2017 Aug;58:561. doi: 10.1016/j.actbio.2017.06.022. Epub 2017 Jul 8. Acta Biomater. 2017. PMID: 28698026 No abstract available.
Abstract
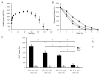
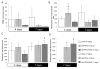
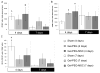
Mesenchymal stromal/stem cells (MSCs) are considered promising cellular therapeutics in the fields of tissue engineering and regenerative medicine. MSCs secrete high concentrations of immunomodulatory cytokines and growth factors, which exert paracrine effects on infiltrating immune and resident cells in the wound microenvironment that could favorably promote healing after acute injury. However, better spatial delivery and improved retention at the site of injury are two factors that could improve the clinical application of MSCs. In this study, we utilized thiol-ene Michael-type addition for rapid encapsulation of MSCs within a gelatin/poly(ethylene glycol) biomatrix. This biomatrix was also applied as a provisional dressing to full thickness wounds in Sprague-Dawley rats. The three-way interaction of MSCs, gelatin/poly(ethylene glycol) biomatrices, and host immune cells and adjacent resident cells in the wound microenvironment favorably modulated wound progression and host response. In this model we observed attenuated immune cell infiltration, lack of foreign giant cell (FBGC) formation, accelerated wound closure and re-epithelialization, as well as enhanced neovascularization and granulation tissue formation by 7 days. The MSC entrapped in the gelatin/poly(ethylene glycol) biomatrix localized cell presentation adjacent to the wound microenvironment and thus mediated the early resolution of inflammatory events and facilitated the proliferative phases in wound healing.
Keywords: Cell-based therapy; Foreign body response; Inflammation; Macrophages; Mesenchymal stem cells.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



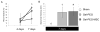



Similar articles
-
Minocycline enhances the mesenchymal stromal/stem cell pro-healing phenotype in triple antimicrobial-loaded hydrogels.Acta Biomater. 2017 Mar 15;51:184-196. doi: 10.1016/j.actbio.2017.01.021. Epub 2017 Jan 7. Acta Biomater. 2017. PMID: 28069512 Free PMC article.
-
Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture.Acta Biomater. 2012 Jul;8(7):2504-16. doi: 10.1016/j.actbio.2012.03.049. Epub 2012 Apr 5. Acta Biomater. 2012. PMID: 22484717 Free PMC article.
-
Induction of mesenchymal stem cell differentiation in the absence of soluble inducer for cutaneous wound regeneration by a chitin nanofiber-based hydrogel.J Tissue Eng Regen Med. 2018 Feb;12(2):e867-e880. doi: 10.1002/term.2400. Epub 2017 May 23. J Tissue Eng Regen Med. 2018. PMID: 28079980
-
Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies.Tissue Eng Part B Rev. 2020 Dec;26(6):555-570. doi: 10.1089/ten.TEB.2019.0351. Epub 2020 May 20. Tissue Eng Part B Rev. 2020. PMID: 32242479 Review.
-
Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy.Stem Cell Res Ther. 2016 Mar 9;7:37. doi: 10.1186/s13287-016-0303-6. Stem Cell Res Ther. 2016. PMID: 26960535 Free PMC article. Review.
Cited by
-
Minocycline enhances the mesenchymal stromal/stem cell pro-healing phenotype in triple antimicrobial-loaded hydrogels.Acta Biomater. 2017 Mar 15;51:184-196. doi: 10.1016/j.actbio.2017.01.021. Epub 2017 Jan 7. Acta Biomater. 2017. PMID: 28069512 Free PMC article.
-
Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering.Gels. 2024 Mar 22;10(4):216. doi: 10.3390/gels10040216. Gels. 2024. PMID: 38667635 Free PMC article. Review.
-
Progress in injectable hydrogels for the treatment of incompressible bleeding: an update.Front Bioeng Biotechnol. 2024 Jan 9;11:1335211. doi: 10.3389/fbioe.2023.1335211. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38264581 Free PMC article. Review.
-
Controlled drug delivery from 3D printed two-photon polymerized poly(ethylene glycol) dimethacrylate devices.Int J Pharm. 2018 Dec 1;552(1-2):217-224. doi: 10.1016/j.ijpharm.2018.09.065. Epub 2018 Sep 27. Int J Pharm. 2018. PMID: 30268853 Free PMC article.
-
Mesenchymal Stem Cells and Exosomes: A Novel Therapeutic Approach for Corneal Diseases.Int J Mol Sci. 2023 Jun 30;24(13):10917. doi: 10.3390/ijms241310917. Int J Mol Sci. 2023. PMID: 37446091 Free PMC article. Review.
References
-
- Grim SA, Clark NM. Management of Infectious Complications in Solid-Organ Transplant Recipients. Nature. 2011;90:333–342. - PubMed
-
- Daley GQ, Scadden DT. Prospects for Cell-Based Therapy. Cell. 2008;132:544–548. - PubMed
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation – how much of the promise has been realized? Nature. 2005;11:605–613. - PubMed
-
- Halme DG, Kessler DA. FDA Regulation of Stem-Cell Based Therapies. New Eng J Med. 2006;355:1730–1735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

