Differential RNA sequence requirement for dengue virus replication in mosquito and mammalian cells
- PMID: 23760236
- PMCID: PMC3754043
- DOI: 10.1128/JVI.00567-13
Differential RNA sequence requirement for dengue virus replication in mosquito and mammalian cells
Abstract
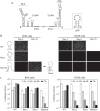
Dengue virus cycles between mosquitoes and humans. Each host provides a different environment for viral replication, imposing different selective pressures. We identified a sequence in the dengue virus genome that is essential for viral replication in mosquito cells but not in mammalian cells. This sequence is located at the viral 3' untranslated region and folds into a small hairpin structure. A systematic mutational analysis using dengue virus infectious clones and reporter viruses allowed the determination of two putative functions in this cis-acting RNA motif, one linked to the structure and the other linked to the nucleotide sequence. We found that single substitutions that did not alter the hairpin structure did not affect dengue virus replication in mammalian cells but abolished replication in mosquito cells. This is the first sequence identified in a flavivirus genome that is exclusively required for viral replication in insect cells.
Figures


Similar articles
-
RNA Structure Duplication in the Dengue Virus 3' UTR: Redundancy or Host Specificity?mBio. 2019 Jan 8;10(1):e02506-18. doi: 10.1128/mBio.02506-18. mBio. 2019. PMID: 30622191 Free PMC article.
-
Functional analysis of dengue virus cyclization sequences located at the 5' and 3'UTRs.Virology. 2008 May 25;375(1):223-35. doi: 10.1016/j.virol.2008.01.014. Epub 2008 Mar 4. Virology. 2008. PMID: 18289628
-
Zika Virus Subgenomic Flavivirus RNA Generation Requires Cooperativity between Duplicated RNA Structures That Are Essential for Productive Infection in Human Cells.J Virol. 2020 Aug 31;94(18):e00343-20. doi: 10.1128/JVI.00343-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32581095 Free PMC article.
-
Functional RNA elements in the dengue virus genome.Viruses. 2011 Sep;3(9):1739-56. doi: 10.3390/v3091739. Epub 2011 Sep 15. Viruses. 2011. PMID: 21994804 Free PMC article. Review.
-
Functional non-coding RNAs derived from the flavivirus 3' untranslated region.Virus Res. 2015 Aug 3;206:53-61. doi: 10.1016/j.virusres.2015.01.026. Epub 2015 Feb 7. Virus Res. 2015. PMID: 25660582 Review.
Cited by
-
High-resolution mapping reveals the mechanism and contribution of genome insertions and deletions to RNA virus evolution.Proc Natl Acad Sci U S A. 2023 Aug;120(31):e2304667120. doi: 10.1073/pnas.2304667120. Epub 2023 Jul 24. Proc Natl Acad Sci U S A. 2023. PMID: 37487061 Free PMC article.
-
Genomic variations in the 3'-termini of Rice stripe virus in the rotation between vector insect and host plant.New Phytol. 2018 Aug;219(3):1085-1096. doi: 10.1111/nph.15246. Epub 2018 Jun 8. New Phytol. 2018. PMID: 29882354 Free PMC article.
-
Structures and Functions of the 3' Untranslated Regions of Positive-Sense Single-Stranded RNA Viruses Infecting Humans and Animals.Front Cell Infect Microbiol. 2020 Aug 27;10:453. doi: 10.3389/fcimb.2020.00453. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974223 Free PMC article. Review.
-
The Role of Noncoding RNA in the Transmission and Pathogenicity of Flaviviruses.Viruses. 2024 Feb 2;16(2):242. doi: 10.3390/v16020242. Viruses. 2024. PMID: 38400018 Free PMC article. Review.
-
Rational design of West Nile virus vaccine through large replacement of 3' UTR with internal poly(A).EMBO Mol Med. 2021 Sep 7;13(9):e14108. doi: 10.15252/emmm.202114108. Epub 2021 Aug 5. EMBO Mol Med. 2021. PMID: 34351689 Free PMC article.
References
-
- Hanley KA, Manlucu LR, Gilmore LE, Blaney JE, Jr, Hanson CT, Murphy BR, Whitehead SS. 2003. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology 312:222–232 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

