Autocrine CCL3 and CCL4 induced by the oncoprotein LMP1 promote Epstein-Barr virus-triggered B cell proliferation
- PMID: 23760235
- PMCID: PMC3754032
- DOI: 10.1128/JVI.00541-13
Autocrine CCL3 and CCL4 induced by the oncoprotein LMP1 promote Epstein-Barr virus-triggered B cell proliferation
Abstract
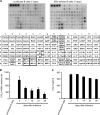
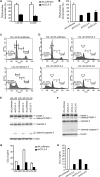
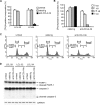
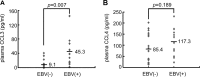
Epstein-Barr virus (EBV) alters the regulation and expression of a variety of cytokines in its host cells to modulate host immune surveillance and facilitate viral persistence. Using cytokine antibody arrays, we found that, in addition to the cytokines reported previously, two chemotactic cytokines, CCL3 and CCL4, were induced in EBV-infected B cells and were expressed at high levels in all EBV-immortalized lymphoblastoid cell lines (LCLs). Furthermore, EBV latent membrane protein 1 (LMP1)-mediated Jun N-terminal protein kinase activation was responsible for upregulation of CCL3 and CCL4. Inhibition of CCL3 and CCL4 in LCLs using a short hairpin RNA approach or by neutralizing antibodies suppressed cell proliferation and caused apoptosis, indicating that autocrine CCL3 and CCL4 are required for LCL survival and growth. Importantly, significant amounts of CCL3 were detected in EBV-positive plasma from immunocompromised patients, suggesting that EBV modulates this chemokine in vivo. This study reveals the regulatory mechanism and a novel function of CCL3 and CCL4 in EBV-infected B cells. CCL3 might be useful as a therapeutic target in EBV-associated lymphoproliferative diseases and malignancies.
Figures




Similar articles
-
Maintenance of Epstein-Barr Virus Latent Status by a Novel Mechanism, Latent Membrane Protein 1-Induced Interleukin-32, via the Protein Kinase Cδ Pathway.J Virol. 2015 Jun;89(11):5968-80. doi: 10.1128/JVI.00168-15. Epub 2015 Mar 25. J Virol. 2015. PMID: 25810549 Free PMC article.
-
The c-Jun N-terminal kinase pathway is critical for cell transformation by the latent membrane protein 1 of Epstein-Barr virus.Virology. 2008 Feb 20;371(2):246-56. doi: 10.1016/j.virol.2007.09.044. Epub 2007 Oct 29. Virology. 2008. PMID: 17967471
-
Dysregulation of Dual-Specificity Phosphatases by Epstein-Barr Virus LMP1 and Its Impact on Lymphoblastoid Cell Line Survival.J Virol. 2020 Jan 31;94(4):e01837-19. doi: 10.1128/JVI.01837-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776277 Free PMC article.
-
The Latent Membrane Protein 1 (LMP1).Curr Top Microbiol Immunol. 2015;391:119-49. doi: 10.1007/978-3-319-22834-1_4. Curr Top Microbiol Immunol. 2015. PMID: 26428373 Review.
-
Latent membrane protein 1 and the B lymphocyte-a complex relationship.Crit Rev Immunol. 2014;34(3):177-98. doi: 10.1615/critrevimmunol.2014010041. Crit Rev Immunol. 2014. PMID: 24941072 Review.
Cited by
-
Stromal ETS2 Regulates Chemokine Production and Immune Cell Recruitment during Acinar-to-Ductal Metaplasia.Neoplasia. 2016 Sep;18(9):541-52. doi: 10.1016/j.neo.2016.07.006. Neoplasia. 2016. PMID: 27659014 Free PMC article.
-
Molecular mechanisms of EBV-driven cell cycle progression and oncogenesis.Med Microbiol Immunol. 2019 Oct;208(5):573-583. doi: 10.1007/s00430-018-0570-1. Epub 2018 Nov 1. Med Microbiol Immunol. 2019. PMID: 30386928 Free PMC article. Review.
-
Mucosal-associated invariant T (MAIT) cells provide B-cell help in vaccinated and subsequently SIV-infected Rhesus Macaques.Sci Rep. 2020 Jun 22;10(1):10060. doi: 10.1038/s41598-020-66964-0. Sci Rep. 2020. PMID: 32572140 Free PMC article.
-
Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment.Adv Cancer Res. 2015;128:263-307. doi: 10.1016/bs.acr.2015.05.001. Epub 2015 Jun 1. Adv Cancer Res. 2015. PMID: 26216636 Free PMC article. Review.
-
Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation.Glia. 2019 Aug;67(8):1571-1597. doi: 10.1002/glia.23630. Epub 2019 Apr 29. Glia. 2019. PMID: 31033049 Free PMC article.
References
-
- Thorley-Lawson DA, Allday MJ. 2008. The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat. Rev. Microbiol. 6:913–924 - PubMed
-
- Dawson CW, Port RJ, Young LS. 2012. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin. Cancer Biol. 22:144–153 - PubMed
-
- Martin D, Gutkind JS. 2008. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene 27(Suppl 2):S31–S42 - PubMed
-
- Jabs WJ, Wagner HJ, Maurmann S, Hennig H, Kreft B. 2002. Inhibition of macrophage inflammatory protein-1α production by Epstein-Barr virus. Blood 99:1512–1516 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

