Nivolumab plus ipilimumab in advanced melanoma
- PMID: 23724867
- PMCID: PMC5698004
- DOI: 10.1056/NEJMoa1302369
Nivolumab plus ipilimumab in advanced melanoma
Erratum in
-
Neoadjuvant PD-1 Blockade in Resectable Lung Cancer; Nivolumab and Ipilimumab in Advanced Melanoma; Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma; Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma; Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma; Rapid Eradication of a Bulky Melanoma Mass with One Dose of Immunotherapy; Genetic Basis for Clinical Response to CTLA-4 Blockade; Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma; Nivolumab plus Ipilimumab in Advanced Melanoma; Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma; Hepatotoxicity with Combination of Vemurafenib and Ipilimumab.N Engl J Med. 2018 Nov 29;379(22):2185. doi: 10.1056/NEJMx180040. Epub 2018 Nov 9. N Engl J Med. 2018. PMID: 31442371 No abstract available.
Abstract
Background: In patients with melanoma, ipilimumab (an antibody against cytotoxic T-lymphocyte-associated antigen 4 [CTLA-4]) prolongs overall survival, and nivolumab (an antibody against the programmed death 1 [PD-1] receptor) produced durable tumor regression in a phase 1 trial. On the basis of their distinct immunologic mechanisms of action and supportive preclinical data, we conducted a phase 1 trial of nivolumab combined with ipilimumab in patients with advanced melanoma.
Methods: We administered intravenous doses of nivolumab and ipilimumab in patients every 3 weeks for 4 doses, followed by nivolumab alone every 3 weeks for 4 doses (concurrent regimen). The combined treatment was subsequently administered every 12 weeks for up to 8 doses. In a sequenced regimen, patients previously treated with ipilimumab received nivolumab every 2 weeks for up to 48 doses.
Results: A total of 53 patients received concurrent therapy with nivolumab and ipilimumab, and 33 received sequenced treatment. The objective-response rate (according to modified World Health Organization criteria) for all patients in the concurrent-regimen group was 40%. Evidence of clinical activity (conventional, unconfirmed, or immune-related response or stable disease for ≥24 weeks) was observed in 65% of patients. At the maximum doses that were associated with an acceptable level of adverse events (nivolumab at a dose of 1 mg per kilogram of body weight and ipilimumab at a dose of 3 mg per kilogram), 53% of patients had an objective response, all with tumor reduction of 80% or more. Grade 3 or 4 adverse events related to therapy occurred in 53% of patients in the concurrent-regimen group but were qualitatively similar to previous experience with monotherapy and were generally reversible. Among patients in the sequenced-regimen group, 18% had grade 3 or 4 adverse events related to therapy and the objective-response rate was 20%.
Conclusions: Concurrent therapy with nivolumab and ipilimumab had a manageable safety profile and provided clinical activity that appears to be distinct from that in published data on monotherapy, with rapid and deep tumor regression in a substantial proportion of patients. (Funded by Bristol-Myers Squibb and Ono Pharmaceutical; ClinicalTrials.gov number, NCT01024231.).
Figures
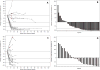
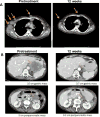
Comment in
-
Combination checkpoint blockade--taking melanoma immunotherapy to the next level.N Engl J Med. 2013 Jul 11;369(2):187-9. doi: 10.1056/NEJMe1305484. Epub 2013 Jun 2. N Engl J Med. 2013. PMID: 23724866 No abstract available.
-
From ASCO-targeted therapies: Anti-PD-1 approaches--important steps forward in metastatic melanoma.Nat Rev Clin Oncol. 2013 Jul;10(7):365. doi: 10.1038/nrclinonc.2013.98. Epub 2013 Jun 11. Nat Rev Clin Oncol. 2013. PMID: 23752732 No abstract available.
Similar articles
-
Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma.N Engl J Med. 2017 Oct 5;377(14):1345-1356. doi: 10.1056/NEJMoa1709684. Epub 2017 Sep 11. N Engl J Med. 2017. PMID: 28889792 Free PMC article. Clinical Trial.
-
Nivolumab and ipilimumab versus ipilimumab in untreated melanoma.N Engl J Med. 2015 May 21;372(21):2006-17. doi: 10.1056/NEJMoa1414428. Epub 2015 Apr 20. N Engl J Med. 2015. PMID: 25891304 Free PMC article. Clinical Trial.
-
Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial.Lancet Oncol. 2016 Jul;17(7):943-955. doi: 10.1016/S1470-2045(16)30126-7. Epub 2016 Jun 4. Lancet Oncol. 2016. PMID: 27269740 Free PMC article. Clinical Trial.
-
Nivolumab: A Review in Advanced Melanoma.Drugs. 2015 Aug;75(12):1413-24. doi: 10.1007/s40265-015-0442-6. Drugs. 2015. PMID: 26220912 Review.
-
Efficacy and safety of anti-PD-1 and anti-PD-1 combined with anti-CTLA-4 immunotherapy to advanced melanoma: A systematic review and meta-analysis of randomized controlled trials.Medicine (Baltimore). 2017 Jun;96(26):e7325. doi: 10.1097/MD.0000000000007325. Medicine (Baltimore). 2017. PMID: 28658143 Free PMC article. Review.
Cited by
-
Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab.Cancer Immunol Res. 2015 Oct;3(10):1185-92. doi: 10.1158/2326-6066.CIR-15-0102. Epub 2015 Jun 22. Cancer Immunol Res. 2015. PMID: 26100356 Free PMC article.
-
Immunotherapy For Ovarian Cancer: Recent Advances And Combination Therapeutic Approaches.Onco Targets Ther. 2020 Jun 26;13:6109-6129. doi: 10.2147/OTT.S205950. eCollection 2020. Onco Targets Ther. 2020. PMID: 32617007 Free PMC article. Review.
-
Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma.Nat Med. 2020 Dec;26(12):1845-1851. doi: 10.1038/s41591-020-1086-y. Epub 2020 Oct 12. Nat Med. 2020. PMID: 33046869 Free PMC article. Clinical Trial.
-
Beyond adjuvants: immunomodulation strategies to enhance T cell immunity.Vaccine. 2015 Jun 8;33 Suppl 2(0 2):B21-8. doi: 10.1016/j.vaccine.2014.12.082. Vaccine. 2015. PMID: 26022562 Free PMC article. Review.
-
Advancing the Minimal Residual Disease Concept in Acute Myeloid Leukemia.Semin Hematol. 2015 Jul;52(3):184-92. doi: 10.1053/j.seminhematol.2015.04.001. Epub 2015 Apr 7. Semin Hematol. 2015. PMID: 26111465 Free PMC article. Review.
References
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
