Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells
- PMID: 23678173
- PMCID: PMC3700181
- DOI: 10.1128/JVI.00506-13
Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells
Abstract
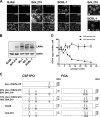
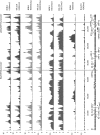
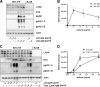
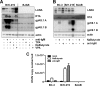
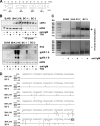
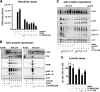
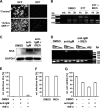
Kaposi's sarcoma-associated herpesvirus (KSHV) is an oncogenic herpesvirus and the cause of Kaposi's sarcoma, primary effusion lymphoma (PEL) and multicentric Castleman's disease. Latently infected B cells are the main reservoir of this virus in vivo, but the nature of the stimuli that lead to its reactivation in B cells is only partially understood. We established stable BJAB cell lines harboring latent KSHV by cell-free infection with recombinant virus carrying a puromycin resistance marker. Our latently infected B cell lines, termed BrK.219, can be reactivated by triggering the B cell receptor (BCR) with antibodies to surface IgM, a stimulus imitating antigen recognition. Using this B cell model system we studied the mechanisms that mediate the reactivation of KSHV in B cells following the stimulation of the BCR and could identify phosphatidylinositol 3-kinase (PI3K) and X-box binding protein 1 (XBP-1) as proteins that play an important role in the BCR-mediated reactivation of latent KSHV.
Figures
Similar articles
-
X box binding protein XBP-1s transactivates the Kaposi's sarcoma-associated herpesvirus (KSHV) ORF50 promoter, linking plasma cell differentiation to KSHV reactivation from latency.J Virol. 2007 Dec;81(24):13578-86. doi: 10.1128/JVI.01663-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928342 Free PMC article.
-
Establishment and maintenance of Kaposi's sarcoma-associated herpesvirus latency in B cells.J Virol. 2005 Nov;79(22):14383-91. doi: 10.1128/JVI.79.22.14383-14391.2005. J Virol. 2005. PMID: 16254372 Free PMC article.
-
Efficient infection of a human B cell line with cell-free Kaposi's sarcoma-associated herpesvirus.J Virol. 2014 Feb;88(3):1748-57. doi: 10.1128/JVI.03063-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257608 Free PMC article.
-
Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus replication.Semin Cancer Biol. 2009 Jun;19(3):153-7. doi: 10.1016/j.semcancer.2009.02.010. Epub 2009 Feb 21. Semin Cancer Biol. 2009. PMID: 19429478 Free PMC article. Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy.J Clin Invest. 2016 Sep 1;126(9):3165-75. doi: 10.1172/JCI84418. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584730 Free PMC article. Review.
-
EphA7 Functions as Receptor on BJAB Cells for Cell-to-Cell Transmission of the Kaposi's Sarcoma-Associated Herpesvirus and for Cell-Free Infection by the Related Rhesus Monkey Rhadinovirus.J Virol. 2019 Jul 17;93(15):e00064-19. doi: 10.1128/JVI.00064-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31118261 Free PMC article.
-
The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside.Virology. 2015 May;479-480:568-77. doi: 10.1016/j.virol.2015.02.040. Epub 2015 Mar 20. Virology. 2015. PMID: 25798530 Free PMC article. Review.
-
Kaposi's sarcoma-associated herpesvirus vIRF2 protein utilizes an IFN-dependent pathway to regulate viral early gene expression.PLoS Pathog. 2019 May 6;15(5):e1007743. doi: 10.1371/journal.ppat.1007743. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31059555 Free PMC article.
-
MxB is an interferon-induced restriction factor of human herpesviruses.Nat Commun. 2018 May 17;9(1):1980. doi: 10.1038/s41467-018-04379-2. Nat Commun. 2018. PMID: 29773792 Free PMC article.
References
-
- Taylor GS, Blackbourn DJ. 2011. Infectious agents in human cancers: lessons in immunity and immunomodulation from gammaherpesviruses EBV and KSHV. Cancer Lett. 305:263–278 - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191 - PubMed
-
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay MF, Clauvel JP, Raphael M, Degos L. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

