Stromal Claudin14-heterozygosity, but not deletion, increases tumour blood leakage without affecting tumour growth
- PMID: 23675413
- PMCID: PMC3652830
- DOI: 10.1371/journal.pone.0062516
Stromal Claudin14-heterozygosity, but not deletion, increases tumour blood leakage without affecting tumour growth
Abstract
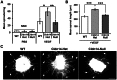
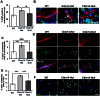
The maintenance of endothelial cell-cell junctions is vital for the control of blood vessel leakage and is known to be important in the growth and maturation of new blood vessels during angiogenesis. Here we have investigated the role of a tight junction molecule, Claudin 14, in tumour blood vessel leakage, angiogenesis and tumour growth. Using syngeneic tumour models our results showed that genetic ablation of Claudin 14 was not sufficient to affect tumour blood vessel morphology or function. However, and surprisingly, Claudin 14-heterozygous mice displayed several blood vessel-related phenotypes including: disruption of ZO-1-positive cell-cell junctions in tumour blood vessels; abnormal distribution of basement membrane laminin around tumour blood vessels; increased intratumoural leakage and decreased intratumoural hypoxia. Additionally, although total numbers of tumour blood vessels were increased in Claudin 14-heterozygous mice, and in VEGF-stimulated angiogenesis ex vivo, the number of lumenated vessels was not changed between genotypes and this correlated with no difference in syngeneic tumour growth between wild-type, Claudin 14-heterozygous and Claudin 14-null mice. Lastly, Claudin 14-heterozygosity, but not complete deficiency, also enhanced endothelial cell proliferation significantly. These data establish a new role for Claudin 14 in the regulation of tumour blood vessel integrity and angiogenesis that is evident only after the partial loss of this molecule in Claudin 14-heterozyous mice but not in Claudin 14-null mice.
Conflict of interest statement
Figures
Similar articles
-
Context dependent role of the CD36--thrombospondin--histidine-rich glycoprotein axis in tumor angiogenesis and growth.PLoS One. 2012;7(7):e40033. doi: 10.1371/journal.pone.0040033. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808089 Free PMC article.
-
PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge.J Cereb Blood Flow Metab. 2012 Apr;32(4):663-75. doi: 10.1038/jcbfm.2011.167. Epub 2011 Nov 30. J Cereb Blood Flow Metab. 2012. PMID: 22126916 Free PMC article.
-
Loss of stromal JUNB does not affect tumor growth and angiogenesis.Int J Cancer. 2014 Mar 15;134(6):1511-6. doi: 10.1002/ijc.28477. Epub 2013 Oct 9. Int J Cancer. 2014. PMID: 24027048
-
Ruffles and spikes: Control of tight junction morphology and permeability by claudins.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183339. doi: 10.1016/j.bbamem.2020.183339. Epub 2020 May 7. Biochim Biophys Acta Biomembr. 2020. PMID: 32389670 Free PMC article. Review.
-
Claudin-4 as therapeutic target in cancer.Arch Biochem Biophys. 2012 Aug 1;524(1):64-70. doi: 10.1016/j.abb.2012.01.009. Epub 2012 Jan 24. Arch Biochem Biophys. 2012. PMID: 22286027 Review.
Cited by
-
Antagonizing Integrin β3 Increases Immunosuppression in Cancer.Cancer Res. 2016 Jun 15;76(12):3484-95. doi: 10.1158/0008-5472.CAN-15-2663. Epub 2016 May 23. Cancer Res. 2016. PMID: 27216180 Free PMC article.
-
Claudin-based barrier differentiation in the colonic epithelial crypt niche involves Hopx/Klf4 and Tcf7l2/Hnf4-α cascades.Tissue Barriers. 2016 Jul 19;4(3):e1214038. doi: 10.1080/21688370.2016.1214038. eCollection 2016 Jul-Sep. Tissue Barriers. 2016. PMID: 27583195 Free PMC article.
-
Mutations in Chromatin Modifier and Ephrin Signaling Genes in Vein of Galen Malformation.Neuron. 2019 Feb 6;101(3):429-443.e4. doi: 10.1016/j.neuron.2018.11.041. Epub 2018 Dec 18. Neuron. 2019. PMID: 30578106 Free PMC article.
-
Emerging multifunctional roles of Claudin tight junction proteins in bone.Endocrinology. 2014 Jul;155(7):2363-76. doi: 10.1210/en.2014-1173. Epub 2014 Apr 23. Endocrinology. 2014. PMID: 24758302 Free PMC article. Review.
-
Platelets and cancer: a casual or causal relationship: revisited.Cancer Metastasis Rev. 2014 Mar;33(1):231-69. doi: 10.1007/s10555-014-9498-0. Cancer Metastasis Rev. 2014. PMID: 24696047 Free PMC article. Review.
References
-
- Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249–257. - PubMed
-
- Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69: 4–10. - PubMed
-
- Lamalice L, Le Boeuf F, Huot J (2007) Endothelial Cell Migration During Angiogenesis. Circ Res 100: 782–794. - PubMed
-
- Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1: 119–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

