Age-specific occurrence of HPV16- and HPV18-related cervical cancer
- PMID: 23632816
- PMCID: PMC4306595
- DOI: 10.1158/1055-9965.EPI-13-0053
Age-specific occurrence of HPV16- and HPV18-related cervical cancer
Abstract
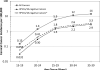
The age-specific occurrence of cervical cancer related to human papillomavirus (HPV) genotypes HPV16 and HPV18, the two targeted by current HPV vaccines, is not well described. We therefore used data from two large, tissue-based HPV genotyping studies of cervical cancer, one conducted in New Mexico (n = 744) and an International study restricted to cancers (n = 1,729) from Europe, North America, and Australia to represent those regions with widely available cervical cancer screening facilities. HPV results were categorized as HPV16- or HPV18-positive (HPV16/18) versus other HPV genotype. We observed a decreasing proportion of HPV16/18-positive cancers with increasing age in the International study (Ptrend < 0.001) and New Mexico study (Ptrend < 0.001). There was no heterogeneity in the relationship between age of diagnosis and the proportion of HPV16/18-positive cancers between studies (P = 0.8). Combining results from the two studies (n = 2,473), the percentages of HPV16/18-positive cases were 77.0% [95% confidence interval (CI): 75.1%-78.9%] for women less than 65 years old and 62.7% [95% confidence interval (CI): 58.4%-66.9%] for women aged 65 and older (P < 0.001). In women who are under the age of 25 and have been vaccinated before becoming sexually active, the cervical cancer incidence is expected to be approximately 3.5 per million by 2020. HPV vaccination against HPV16/18 may have a greater impact on cervical cancers in women under 65 than in women aged 65 and older. These data will inform the age-specific impact of HPV vaccination and its integration with cervical cancer screening activities.
Figures

Similar articles
-
Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States.J Natl Cancer Inst. 2009 Apr 1;101(7):475-87. doi: 10.1093/jnci/djn510. Epub 2009 Mar 24. J Natl Cancer Inst. 2009. PMID: 19318628 Free PMC article.
-
Looking beyond human papillomavirus (HPV) genotype 16 and 18: Defining HPV genotype distribution in cervical cancers in Australia prior to vaccination.Int J Cancer. 2017 Oct 15;141(8):1576-1584. doi: 10.1002/ijc.30871. Epub 2017 Jul 14. Int J Cancer. 2017. PMID: 28677147
-
HIGH-RISK HPV testing as the primary screening method in an organized regional screening program for cervical cancer: the value of HPV16 and HPV18 genotyping?APMIS. 2019 Nov;127(11):710-716. doi: 10.1111/apm.12990. Epub 2019 Sep 11. APMIS. 2019. PMID: 31403733
-
Population-based prevalence of cervical infection with human papillomavirus genotypes 16 and 18 and other high risk types in Tlaxcala, Mexico.BMC Infect Dis. 2016 Sep 1;16(1):461. doi: 10.1186/s12879-016-1782-x. BMC Infect Dis. 2016. PMID: 27585544 Free PMC article.
-
Age-specific prevalence of HPV16/18 genotypes in cervical cancer: A systematic review and meta-analysis.Int J Cancer. 2016 Jun 15;138(12):2795-803. doi: 10.1002/ijc.29959. Epub 2016 Jan 11. Int J Cancer. 2016. PMID: 26661889 Review.
Cited by
-
Predictable changes in the accuracy of human papillomavirus tests after vaccination: review with implications for performance monitoring in cervical screening.Br J Cancer. 2024 May;130(11):1733-1743. doi: 10.1038/s41416-024-02681-z. Epub 2024 Apr 13. Br J Cancer. 2024. PMID: 38615108 Free PMC article. Review.
-
Clinical impact of age‑specific distribution of combination patterns of cytology and high‑risk HPV status on cervical intraepithelial neoplasia grade 2 or more.Oncol Lett. 2023 Jul 20;26(3):384. doi: 10.3892/ol.2023.13970. eCollection 2023 Sep. Oncol Lett. 2023. PMID: 37559589 Free PMC article.
-
Distribution of human papillomavirus genotypes by severity of cervical lesions in HPV screened positive women from the ESTAMPA study in Latin America.PLoS One. 2022 Jul 29;17(7):e0272205. doi: 10.1371/journal.pone.0272205. eCollection 2022. PLoS One. 2022. PMID: 35905130 Free PMC article.
-
Comparison of Seegene Anyplex II HPV28 assay with BD Onclarity HPV assay for human papillomavirus genotyping.PLoS One. 2022 Jul 8;17(7):e0267836. doi: 10.1371/journal.pone.0267836. eCollection 2022. PLoS One. 2022. PMID: 35802570 Free PMC article.
-
Cervical cancer prevention and control in women living with human immunodeficiency virus.CA Cancer J Clin. 2021 Nov;71(6):505-526. doi: 10.3322/caac.21696. Epub 2021 Sep 9. CA Cancer J Clin. 2021. PMID: 34499351 Free PMC article. Review.
References
-
- de SS, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. - PubMed
-
- Romanowski B, de Borba PC, Naud PS, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374(9706):1975–85. - PubMed
-
- Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. - PubMed
-
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. - PubMed
-
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

