Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet
- PMID: 23632634
- PMCID: PMC4116354
- DOI: 10.1152/ajpendo.00584.2012
Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet
Erratum in
- Am J Physiol Endocrinol Metab. 2013 Oct 15;305(8):E1048
Abstract
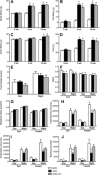
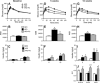
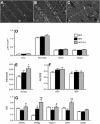
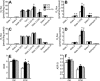
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) enhance insulin sensitivity and glucose homeostasis in rodent models of insulin resistance. These beneficial effects have been linked with anti-inflammatory properties, but emerging data suggest that the mechanisms may also converge on mitochondria. We evaluated the influence of dietary n-3 PUFAs on mitochondrial physiology and muscle lipid metabolites in the context of high-fat diet (HFD) in mice. Mice were fed control diets (10% fat), HFD (60% fat), or HFD with fish oil (HFD+FO, 3.4% kcal from n-3 PUFAs) for 10 wk. Body mass and fat mass increased similarly in HFD and HFD+FO, but n-3 PUFAs attenuated the glucose intolerance that developed with HFD and increased expression of genes that regulate glucose metabolism in skeletal muscle. Despite similar muscle triglyceride levels in HFD and HFD+FO, long-chain acyl-CoAs and ceramides were lower in the presence of fish oil. Mitochondrial abundance and oxidative capacity were similarly increased in HFD and HFD+FO compared with controls. Hydrogen peroxide production was similarly elevated in HFD and HFD+FO in isolated mitochondria but not in permeabilized muscle fibers, likely due to increased activity and expression of catalase. These results support a hypothesis that n-3 PUFAs protect glucose tolerance, in part by preventing the accumulation of bioactive lipid mediators that interfere with insulin action. Furthermore, the respiratory function of skeletal muscle mitochondria does not appear to be a major factor in sphingolipid accumulation, glucose intolerance, or the protective effects of n-3 PUFAs.
Keywords: ceramide; essential fatty acids; fish oil; high-fat diet; insulin sensitivity; long-chain acyl-coenzyme A; mitochondria; omega-3 fatty acids; polyunsaturated fatty acids; sphingolipid.
Figures
Similar articles
-
Sexual dimorphism in the effects of maternal adipose tissue growth hormone receptor deficiency on offspring metabolic health.Biol Sex Differ. 2024 Dec 2;15(1):98. doi: 10.1186/s13293-024-00676-2. Biol Sex Differ. 2024. PMID: 39623508 Free PMC article.
-
A Maternal Western-Style Diet Impairs Skeletal Muscle Lipid Metabolism in Adolescent Japanese Macaques.Diabetes. 2023 Dec 1;72(12):1766-1780. doi: 10.2337/db23-0289. Diabetes. 2023. PMID: 37725952 Free PMC article.
-
Systemic inhibition of de novo purine biosynthesis prevents weight gain and improves metabolic health by increasing thermogenesis and decreasing food intake.bioRxiv [Preprint]. 2024 Nov 1:2024.10.28.620705. doi: 10.1101/2024.10.28.620705. bioRxiv. 2024. PMID: 39553975 Free PMC article. Preprint.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
The Role of Ceramides in Insulin Resistance.Front Endocrinol (Lausanne). 2019 Aug 21;10:577. doi: 10.3389/fendo.2019.00577. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31496996 Free PMC article. Review.
-
Adipose Tissue Mitochondrial Factors Profile after Dietary Bioactive Compound Weight Reduction Treatments in a Mice Obesity Model.Int J Mol Sci. 2019 Nov 22;20(23):5870. doi: 10.3390/ijms20235870. Int J Mol Sci. 2019. PMID: 31771102 Free PMC article.
-
The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats.Nutrients. 2019 Apr 12;11(4):835. doi: 10.3390/nu11040835. Nutrients. 2019. PMID: 31013835 Free PMC article.
-
Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways.Metab Brain Dis. 2014 Mar;29(1):19-36. doi: 10.1007/s11011-013-9435-x. Epub 2013 Sep 10. Metab Brain Dis. 2014. PMID: 24557875
-
Individual differences in EPA and DHA content of Atlantic salmon are associated with gene expression of key metabolic processes.Sci Rep. 2019 Mar 7;9(1):3889. doi: 10.1038/s41598-019-40391-2. Sci Rep. 2019. PMID: 30846825 Free PMC article.
References
-
- Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 - PubMed
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 - PMC - PubMed
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009 - PMC - PubMed
-
- Beha A, Juretschke HP, Kuhlmann J, Neumann-Haefelin C, Belz U, Gerl M, Kramer W, Roden M, Herling AW. Muscle type-specific fatty acid metabolism in insulin resistance: an integrated in vivo study in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 290: E989–E997, 2006 - PubMed
-
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, Baumruker T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 17: 1203–1217, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

