TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease
- PMID: 23566200
- PMCID: PMC3634536
- DOI: 10.1111/imm.12045
TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn's disease
Abstract
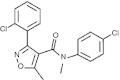
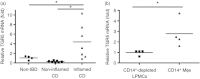
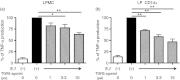
Bile acids (BAs) play important roles not only in lipid metabolism, but also in signal transduction. TGR5, a transmembrane receptor of BAs, is an immunomodulative factor, but its detailed mechanism remains unclear. Here, we aimed to delineate how BAs operate in immunological responses via the TGR5 pathway in human mononuclear cell lineages. We examined TGR5 expression in human peripheral blood monocytes, several types of in vitro differentiated macrophages (Mϕs) and dendritic cells. Mϕs differentiated with macrophage colony-stimulating factor and interferon-γ (Mγ-Mϕs), which are similar to the human intestinal lamina propria CD14(+) Mϕs that contribute to Crohn's disease (CD) pathogenesis by production of pro-inflammatory cytokines, highly expressed TGR5 compared with any other type of differentiated Mϕ and dendritic cells. We also showed that a TGR5 agonist and two types of BAs, deoxycholic acid and lithocholic acid, could inhibit tumour necrosis factor-α production in Mγ-Mϕs stimulated by commensal bacterial antigen or lipopolysaccharide. This inhibitory effect was mediated by the TGR5-cAMP pathway to induce phosphorylation of c-Fos that regulated nuclear factor-κB p65 activation. Next, we analysed TGR5 levels in lamina propria mononuclear cells (LPMCs) obtained from the intestinal mucosa of patients with CD. Compared with non-inflammatory bowel disease, inflamed CD LPMCs contained more TGR5 transcripts. Among LPMCs, isolated CD14(+) intestinal Mϕs from patients with CD expressed TGR5. In isolated intestinal CD14(+) Mϕs, a TGR5 agonist could inhibit tumour necrosis factor-α production. These results indicate that TGR5 signalling may have the potential to modulate immune responses in inflammatory bowel disease.
© 2013 Blackwell Publishing Ltd.
Figures
Similar articles
-
β-(1,3)-Glucan derived from Candida albicans induces inflammatory cytokines from macrophages and lamina propria mononuclear cells derived from patients with Crohn's disease.Intest Res. 2018 Jul;16(3):384-392. doi: 10.5217/ir.2018.16.3.384. Epub 2018 Jul 27. Intest Res. 2018. PMID: 30090037 Free PMC article.
-
Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with Crohn's disease.Gastroenterology. 2014 May;146(5):1278-88.e1-2. doi: 10.1053/j.gastro.2014.01.057. Epub 2014 Feb 4. Gastroenterology. 2014. PMID: 24503130
-
Reduced numbers of mucosal DR(int) macrophages and increased numbers of CD103(+) dendritic cells during anti-TNF-α treatment in patients with Crohn's disease.Scand J Gastroenterol. 2016;51(6):692-9. doi: 10.3109/00365521.2015.1134649. Epub 2016 Jan 19. Scand J Gastroenterol. 2016. PMID: 26784676
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Role of the IL23/IL17 Pathway in Crohn's Disease.Front Immunol. 2021 Mar 30;12:622934. doi: 10.3389/fimmu.2021.622934. eCollection 2021. Front Immunol. 2021. PMID: 33859636 Free PMC article. Review.
Cited by
-
Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract.J Physiol. 2014 Jul 15;592(14):2943-50. doi: 10.1113/jphysiol.2014.271155. Epub 2014 Mar 10. J Physiol. 2014. PMID: 24614746 Free PMC article. Review.
-
Microbial Metabolite Dysbiosis and Colorectal Cancer.Gut Liver. 2023 Mar 15;17(2):190-203. doi: 10.5009/gnl220260. Epub 2023 Jan 12. Gut Liver. 2023. PMID: 36632785 Free PMC article. Review.
-
Food as Treatment of Inflammatory Bowel Diseases.Infect Immun. 2022 May 19;90(5):e0058321. doi: 10.1128/iai.00583-21. Epub 2022 May 11. Infect Immun. 2022. PMID: 35323020 Free PMC article. Review.
-
Contributions of bile acids to gastrointestinal physiology as receptor agonists and modifiers of ion channels.Am J Physiol Gastrointest Liver Physiol. 2022 Feb 1;322(2):G201-G222. doi: 10.1152/ajpgi.00125.2021. Epub 2021 Nov 10. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34755536 Free PMC article.
-
Bacterial-Induced Blood Pressure Reduction: Mechanisms for the Treatment of Hypertension via the Gut.Front Cardiovasc Med. 2021 Aug 13;8:721393. doi: 10.3389/fcvm.2021.721393. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34485420 Free PMC article. Review.
References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. - PubMed
-
- Kamada N, Hisamatsu T, Okamoto S, et al. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–8. - PubMed
-
- Takada Y, Hisamatsu T, Kamada N, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

