Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure
- PMID: 23562075
- PMCID: PMC5967396
- DOI: 10.1016/j.cmet.2013.03.002
Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure
Abstract
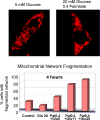
Mitochondrial fusion, fission, and mitophagy form an essential axis of mitochondrial quality control. However, quality control might not be the only task carried out by mitochondrial dynamics. Recent studies link mitochondrial dynamics to the balance between energy demand and nutrient supply, suggesting changes in mitochondrial architecture as a mechanism for bioenergetic adaptation to metabolic demands. By favoring either connected or fragmented architectures, mitochondrial dynamics regulates bioenergetic efficiency and energy expenditure. Placement of bioenergetic adaptation and quality control as competing tasks of mitochondrial dynamics might provide a new mechanism, linking excess nutrient environment to progressive mitochondrial dysfunction, common to age-related diseases.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



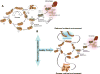
Similar articles
-
Emerging Concepts in Diabetes: Mitochondrial Dynamics and Glucose Homeostasis.Curr Diabetes Rev. 2017;13(4):370-385. doi: 10.2174/1573399812666151012115229. Curr Diabetes Rev. 2017. PMID: 26456359 Review.
-
Bioenergetic role of mitochondrial fusion and fission.Biochim Biophys Acta. 2012 Oct;1817(10):1833-8. doi: 10.1016/j.bbabio.2012.02.033. Epub 2012 Mar 5. Biochim Biophys Acta. 2012. PMID: 22409868
-
Metabolic regulation of mitochondrial morphologies in pancreatic beta cells: coupling of bioenergetics and mitochondrial dynamics.Commun Biol. 2024 Oct 5;7(1):1267. doi: 10.1038/s42003-024-06955-3. Commun Biol. 2024. PMID: 39369076 Free PMC article.
-
Mitochondrial dynamics and morphology in beta-cells.Best Pract Res Clin Endocrinol Metab. 2012 Dec;26(6):725-38. doi: 10.1016/j.beem.2012.05.004. Epub 2012 Jul 26. Best Pract Res Clin Endocrinol Metab. 2012. PMID: 23168275 Free PMC article. Review.
-
Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications.Redox Biol. 2017 Apr;11:637-645. doi: 10.1016/j.redox.2017.01.013. Epub 2017 Jan 16. Redox Biol. 2017. PMID: 28131082 Free PMC article. Review.
Cited by
-
Mitochondrial Function in Enamel Development.Front Physiol. 2020 May 29;11:538. doi: 10.3389/fphys.2020.00538. eCollection 2020. Front Physiol. 2020. PMID: 32547417 Free PMC article.
-
The cellular and molecular progression of mitochondrial dysfunction induced by 2,4-dinitrophenol in developing zebrafish embryos.Differentiation. 2015 Mar-Apr;89(3-4):51-69. doi: 10.1016/j.diff.2015.01.001. Epub 2015 Mar 12. Differentiation. 2015. PMID: 25771346 Free PMC article.
-
Mechanism of PKM2 affecting cancer immunity and metabolism in Tumor Microenvironment.J Cancer. 2021 Apr 24;12(12):3566-3574. doi: 10.7150/jca.54430. eCollection 2021. J Cancer. 2021. PMID: 33995634 Free PMC article. Review.
-
Critical requirement of SOS1 RAS-GEF function for mitochondrial dynamics, metabolism, and redox homeostasis.Oncogene. 2021 Jul;40(27):4538-4551. doi: 10.1038/s41388-021-01886-3. Epub 2021 Jun 12. Oncogene. 2021. PMID: 34120142 Free PMC article.
-
Restricting bioenergetic efficiency enhances longevity and mitochondrial redox capacity in Drosophila melanogaster.Aging Cell. 2024 May;23(5):e14107. doi: 10.1111/acel.14107. Epub 2024 Feb 11. Aging Cell. 2024. PMID: 38343281 Free PMC article.
References
-
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

