Correlations between pharmacokinetics of IgG antibodies in primates vs. FcRn-transgenic mice reveal a rodent model with predictive capabilities
- PMID: 23549129
- PMCID: PMC4169033
- DOI: 10.4161/mabs.23836
Correlations between pharmacokinetics of IgG antibodies in primates vs. FcRn-transgenic mice reveal a rodent model with predictive capabilities
Abstract
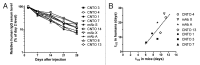
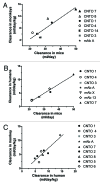
Transgenic mice expressing human neonatal Fc receptor (FcRn) instead of mouse FcRn are available for IgG antibody pharmacokinetic (PK) studies. Given the interest in a rodent model that offers reliable predictions of antibody PK in monkeys and humans, we set out to test whether the PK of IgG antibodies in such mice correlated with the PK of the same antibodies in primates. We began by using a single research antibody to study the influence of: (1) different transgenic mouse lines that differ in FcRn transgene expression; (2) homozygous vs. hemizygous FcRn transgenic mice; (3) the presence vs. absence of coinjected high-dose human intravenous immunoglobulin (IVIG), and (4) the presence vs. absence of coinjected high-dose human serum albumin (HSA). Results of those studies suggested that use of hemizygous Tg32 mice (Tg32 hemi) not treated with IVIG or HSA offered potential as a predictive model for PK in humans. Mouse PK studies were then done under those conditions with a panel of test antibodies whose PK in mice and primates is not significantly affected by target binding, and for which monkey or human PK data were readily available. Results from the studies revealed significant correlations between terminal half-life or clearance values observed in the mice and the corresponding values reported in humans. A significant relationship in clearance values between mice and monkeys was also observed. These correlations suggest that the Tg32 hemi mouse model, which is both convenient and cost-effective, can offer value in predicting antibody half-life and clearance in primates.
Keywords: FcRn; HSA; IVIG; PK; Tg276; Tg32; antibody; correlations; primates; transgenic mice.
Figures



Similar articles
-
Utility of a human FcRn transgenic mouse model in drug discovery for early assessment and prediction of human pharmacokinetics of monoclonal antibodies.MAbs. 2016 Aug-Sep;8(6):1064-78. doi: 10.1080/19420862.2016.1193660. Epub 2016 May 27. MAbs. 2016. PMID: 27232760 Free PMC article.
-
Pharmacokinetics of novel Fc-engineered monoclonal and multispecific antibodies in cynomolgus monkeys and humanized FcRn transgenic mouse models.MAbs. 2020 Jan-Dec;12(1):1829337. doi: 10.1080/19420862.2020.1829337. MAbs. 2020. PMID: 33079615 Free PMC article.
-
Linear pharmacokinetic parameters for monoclonal antibodies are similar within a species and across different pharmacological targets: A comparison between human, cynomolgus monkey and hFcRn Tg32 transgenic mouse using a population-modeling approach.MAbs. 2018 Jul;10(5):751-764. doi: 10.1080/19420862.2018.1462429. Epub 2018 May 14. MAbs. 2018. PMID: 29634430 Free PMC article.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering.BioDrugs. 2021 Mar;35(2):147-157. doi: 10.1007/s40259-021-00471-0. Epub 2021 Feb 19. BioDrugs. 2021. PMID: 33608823 Free PMC article. Review.
Cited by
-
A Minimal Physiologically Based Pharmacokinetic Model with a Nested Endosome Compartment for Novel Engineered Antibodies.AAPS J. 2018 Mar 14;20(3):48. doi: 10.1208/s12248-017-0183-4. AAPS J. 2018. PMID: 29541870 Free PMC article.
-
Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice.MAbs. 2018 Jul;10(5):803-813. doi: 10.1080/19420862.2018.1458808. Epub 2018 May 9. MAbs. 2018. PMID: 29621428 Free PMC article.
-
Characterization and screening of IgG binding to the neonatal Fc receptor.MAbs. 2014 Jul-Aug;6(4):928-42. doi: 10.4161/mabs.28744. Epub 2014 Apr 7. MAbs. 2014. PMID: 24802048 Free PMC article.
-
Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients.Antibodies (Basel). 2019 Jan 1;8(1):3. doi: 10.3390/antib8010003. Antibodies (Basel). 2019. PMID: 31544809 Free PMC article. Review.
-
Pharmacokinetic and Pharmacodynamic Considerations in the Design of Therapeutic Antibodies.Clin Transl Sci. 2019 Mar;12(2):130-139. doi: 10.1111/cts.12597. Epub 2018 Dec 27. Clin Transl Sci. 2019. PMID: 30414357 Free PMC article. Review.
References
-
- Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–56. - PubMed
-
- Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–69. doi: 10.1093/intimm/dxl110. - DOI - PubMed
-
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
