A pathogenic picornavirus acquires an envelope by hijacking cellular membranes
- PMID: 23542590
- PMCID: PMC3631468
- DOI: 10.1038/nature12029
A pathogenic picornavirus acquires an envelope by hijacking cellular membranes
Abstract
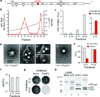
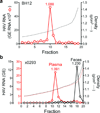
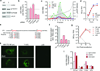
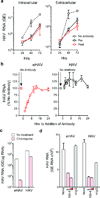
Animal viruses are broadly categorized structurally by the presence or absence of an envelope composed of a lipid-bilayer membrane, attributes that profoundly affect stability, transmission and immune recognition. Among those lacking an envelope, the Picornaviridae are a large and diverse family of positive-strand RNA viruses that includes hepatitis A virus (HAV), an ancient human pathogen that remains a common cause of enterically transmitted hepatitis. HAV infects in a stealth-like manner and replicates efficiently in the liver. Virus-specific antibodies appear only after 3-4 weeks of infection, and typically herald its resolution. Although unexplained mechanistically, both anti-HAV antibody and inactivated whole-virus vaccines prevent disease when administered as late as 2 weeks after exposure, when virus replication is well established in the liver. Here we show that HAV released from cells is cloaked in host-derived membranes, thereby protecting the virion from antibody-mediated neutralization. These enveloped viruses ('eHAV') resemble exosomes, small vesicles that are increasingly recognized to be important in intercellular communications. They are fully infectious, sensitive to extraction with chloroform, and circulate in the blood of infected humans. Their biogenesis is dependent on host proteins associated with endosomal-sorting complexes required for transport (ESCRT), namely VPS4B and ALIX. Whereas the hijacking of membranes by HAV facilitates escape from neutralizing antibodies and probably promotes virus spread within the liver, anti-capsid antibodies restrict replication after infection with eHAV, suggesting a possible explanation for prophylaxis after exposure. Membrane hijacking by HAV blurs the classic distinction between 'enveloped' and 'non-enveloped' viruses and has broad implications for mechanisms of viral egress from infected cells as well as host immune responses.
Figures
Comment in
-
Viral pathogenesis: Cloak and dagger.Nat Rev Microbiol. 2013 Jun;11(6):360. doi: 10.1038/nrmicro3026. Epub 2013 Apr 16. Nat Rev Microbiol. 2013. PMID: 23588217 No abstract available.
-
Hepatitis virus hijacks shuttle: exosome-like vesicles provide protection against neutralizing antibodies.Hepatology. 2014 Jun;59(6):2416-8. doi: 10.1002/hep.26943. Epub 2014 Apr 14. Hepatology. 2014. PMID: 24273053 No abstract available.
Similar articles
-
TIM1 (HAVCR1) Is Not Essential for Cellular Entry of Either Quasi-enveloped or Naked Hepatitis A Virions.mBio. 2017 Sep 5;8(5):e00969-17. doi: 10.1128/mBio.00969-17. mBio. 2017. PMID: 28874468 Free PMC article.
-
Redundant Late Domain Functions of Tandem VP2 YPX3L Motifs in Nonlytic Cellular Egress of Quasi-enveloped Hepatitis A Virus.J Virol. 2018 Nov 12;92(23):e01308-18. doi: 10.1128/JVI.01308-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232181 Free PMC article.
-
Peek-a-boo: membrane hijacking and the pathogenesis of viral hepatitis.Trends Microbiol. 2014 Feb;22(2):59-64. doi: 10.1016/j.tim.2013.10.005. Epub 2013 Nov 19. Trends Microbiol. 2014. PMID: 24268716 Free PMC article.
-
Quasi-enveloped hepatitis virus assembly and release.Adv Virus Res. 2020;108:315-336. doi: 10.1016/bs.aivir.2020.08.004. Epub 2020 Sep 28. Adv Virus Res. 2020. PMID: 33837720 Review.
-
[Hepatitis A virus infection. A review].Praxis (Bern 1994). 2003 Oct 1;92(40):1659-73. doi: 10.1024/0369-8394.92.40.1659. Praxis (Bern 1994). 2003. PMID: 14579471 Review. German.
Cited by
-
A genome-wide CRISPR screen identifies UFMylation and TRAMP-like complexes as host factors required for hepatitis A virus infection.Cell Rep. 2021 Mar 16;34(11):108859. doi: 10.1016/j.celrep.2021.108859. Cell Rep. 2021. PMID: 33730579 Free PMC article.
-
Exosomes as Conduits: Facilitating Hepatitis B Virus-Independent Hepatitis D Virus Transmission and Propagation in Hepatocytes.Viruses. 2024 May 22;16(6):825. doi: 10.3390/v16060825. Viruses. 2024. PMID: 38932118 Free PMC article.
-
Tropism of human pegivirus (formerly known as GB virus C/hepatitis G virus) and host immunomodulation: insights into a highly successful viral infection.J Gen Virol. 2015 Jul;96(Pt 7):1521-32. doi: 10.1099/vir.0.000086. Epub 2015 Feb 9. J Gen Virol. 2015. PMID: 25667328 Free PMC article. Review.
-
Iminosugar Glucosidase Inhibitors Reduce Hepatic Inflammation in Hepatitis A Virus-Infected Ifnar1-/- Mice.J Virol. 2021 May 10;95(11):e0005821. doi: 10.1128/JVI.00058-21. Epub 2021 May 10. J Virol. 2021. PMID: 33692213 Free PMC article.
-
Zika Virus-Infected Monocyte Exosomes Mediate Cell-to-Cell Viral Transmission.Cells. 2024 Jan 12;13(2):144. doi: 10.3390/cells13020144. Cells. 2024. PMID: 38247836 Free PMC article.
References
-
- Harrison SC. In: Fields Virology. Knipe DM, et al., editors. Lippincott Williams & Wilkins; 2007. pp. 59–98. Ch. 3.
-
- Feng Z, Lemon SM. In: The Picornaviruses. Ehrenfeld E, Domingo E, Roos RP, editors. Washington, DC: ASM Press; 2010. pp. 383–396. Ch. 25.
-
- Lemon SM. Type A viral hepatitis: new developments in an old disease. New Engl. J. Med. 1985;313:1059–1067. - PubMed
-
- Martin A, Lemon SM. In: Hepatitis Viruses. Ou J, editor. Kluwer Academic Publishers; 2002. pp. 23–50.
Additional References
-
- Taylor KL, et al. Attenuation phenotype of a cell culture-adapted variant of hepatitis A virus (HM175/p16) in susceptible new world owl monkeys. J Infect Dis. 1993;168:592–601. - PubMed
-
- Lemon SM, et al. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J. Med. Virol. 1982;10:25–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

