Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine
- PMID: 23508958
- PMCID: PMC3642326
- DOI: 10.1074/jbc.M113.450072
Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine
Abstract
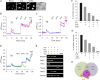
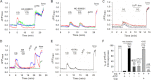
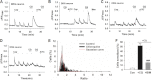
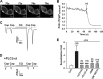
The sensations of pain, itch, and cold often interact with each other. Pain inhibits itch, whereas cold inhibits both pain and itch. TRPV1 and TRPA1 channels transduce pain and itch, whereas TRPM8 transduces cold. The pruritogen chloroquine (CQ) was reported to excite TRPA1, leading to the sensation of itch. It is unclear how CQ excites and modulates TRPA1(+), TRPV1(+), and TRPM8(+) neurons and thus affects the sensations of pain, itch, and cold. Here, we show that only 43% of CQ-excited dorsal root ganglion neurons expressed TRPA1; as expected, the responses of these neurons were completely prevented by the TRPA1 antagonist HC-030031. The remaining 57% of CQ-excited neurons did not express TRPA1, and excitation was not prevented by either a TRPA1 or TRPV1 antagonist but was prevented by the general transient receptor potential canonical (TRPC) channel blocker BTP2 and the selective TRPC3 inhibitor Pyr3. Furthermore, CQ caused potent sensitization of TRPV1 in 51.9% of TRPV1(+) neurons and concomitant inhibition of TRPM8 in 48.8% of TRPM8(+) dorsal root ganglion neurons. Sensitization of TRPV1 is caused mainly by activation of the phospholipase C-PKC pathway following activation of the CQ receptor MrgprA3. By contrast, inhibition of TRPM8 is caused by a direct action of activated Gαq independent of the phospholipase C pathway. Our data suggest the involvement of the TRPC3 channel acting together with TRPA1 to mediate CQ-induced itch. CQ not only elicits itch by directly exciting itch-encoding neurons but also exerts previously unappreciated widespread actions on pain-, itch-, and cold-sensing neurons, leading to enhanced pain and itch.
Keywords: Calcium Imaging; Chloroquine; Electrophysiology; G Protein-coupled Receptor (GPCR); Itch; Pain; Sensory Transduction; Signal Transduction; TRP Channels.
Figures


Similar articles
-
TRPV1 and TRPA1 Channels Are Both Involved Downstream of Histamine-Induced Itch.Biomolecules. 2021 Aug 6;11(8):1166. doi: 10.3390/biom11081166. Biomolecules. 2021. PMID: 34439832 Free PMC article.
-
Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors.J Comp Neurol. 2005 Dec 26;493(4):596-606. doi: 10.1002/cne.20794. J Comp Neurol. 2005. PMID: 16304633
-
Nociceptive transient receptor potential ankyrin 1 (TRPA1) in sensory neurons are targets of the antifungal drug econazole.BMC Pharmacol Toxicol. 2024 Aug 21;25(1):53. doi: 10.1186/s40360-024-00779-x. BMC Pharmacol Toxicol. 2024. PMID: 39169383 Free PMC article.
-
Thermosensation and TRP Channels.Adv Exp Med Biol. 2024;1461:3-13. doi: 10.1007/978-981-97-4584-5_1. Adv Exp Med Biol. 2024. PMID: 39289270 Review.
-
Itching, chloroquine, and malaria: a review of recent molecular and neuroscience advances and their contribution to mechanistic understanding and therapeutics of chronic non-histaminergic pruritus.Int J Dermatol. 2019 Aug;58(8):880-891. doi: 10.1111/ijd.14252. Epub 2018 Oct 26. Int J Dermatol. 2019. PMID: 30362504 Review.
Cited by
-
Mouse model of imiquimod-induced psoriatic itch.Pain. 2016 Nov;157(11):2536-2543. doi: 10.1097/j.pain.0000000000000674. Pain. 2016. PMID: 27437787 Free PMC article.
-
Direct Gαq Gating Is the Sole Mechanism for TRPM8 Inhibition Caused by Bradykinin Receptor Activation.Cell Rep. 2019 Jun 18;27(12):3672-3683.e4. doi: 10.1016/j.celrep.2019.05.080. Cell Rep. 2019. PMID: 31216483 Free PMC article.
-
Activation of TRPC channels contributes to OA-NO2-induced responses in guinea-pig dorsal root ganglion neurons.J Physiol. 2014 Oct 1;592(19):4297-312. doi: 10.1113/jphysiol.2014.271783. Epub 2014 Aug 15. J Physiol. 2014. PMID: 25128576 Free PMC article.
-
The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging.Exp Brain Res. 2018 Aug;236(8):2231-2244. doi: 10.1007/s00221-018-5299-y. Epub 2018 May 29. Exp Brain Res. 2018. PMID: 29845449
-
The Specification and Maturation of Nociceptive Neurons from Human Embryonic Stem Cells.Sci Rep. 2015 Nov 19;5:16821. doi: 10.1038/srep16821. Sci Rep. 2015. PMID: 26581770 Free PMC article.
References
-
- Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., Koltzenburg M., Basbaum A. I., Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 - PubMed
-
- Davis J. B., Gray J., Gunthorpe M. J., Hatcher J. P., Davey P. T., Overend P., Harries M. H., Latcham J., Clapham C., Atkinson K., Hughes S. A., Rance K., Grau E., Harper A. J., Pugh P. L., Rogers D. C., Bingham S., Randall A., Sheardown S. A. (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187 - PubMed
-
- Kwan K. Y., Allchorne A. J., Vollrath M. A., Christensen A. P., Zhang D. S., Woolf C. J., Corey D. P. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 - PubMed
-
- Vetter I., Touska F., Hess A., Hinsbey R., Sattler S., Lampert A., Sergejeva M., Sharov A., Collins L. S., Eberhardt M., Engel M., Cabot P. J., Wood J. N., Vlachová V., Reeh P. W., Lewis R. J., Zimmermann K. (2012) Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 31, 3795–3808 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

