Retrotransposition of gene transcripts leads to structural variation in mammalian genomes
- PMID: 23497673
- PMCID: PMC3663115
- DOI: 10.1186/gb-2013-14-3-r22
Retrotransposition of gene transcripts leads to structural variation in mammalian genomes
Abstract
Background: Retroposed processed gene transcripts are an important source of material for new gene formation on evolutionary timescales. Most prior work on gene retrocopy discovery compared copies in reference genome assemblies to their source genes. Here, we explore gene retrocopy insertion polymorphisms (GRIPs) that are present in the germlines of individual humans, mice, and chimpanzees, and we identify novel gene retrocopy insertions in cancerous somatic tissues that are absent from patient-matched non-cancer genomes.
Results: Through analysis of whole-genome sequence data, we found evidence for 48 GRIPs in the genomes of one or more humans sequenced as part of the 1,000 Genomes Project and The Cancer Genome Atlas, but which were not in the human reference assembly. Similarly, we found evidence for 755 GRIPs at distinct locations in one or more of 17 inbred mouse strains but which were not in the mouse reference assembly, and 19 GRIPs across a cohort of 10 chimpanzee genomes, which were not in the chimpanzee reference genome assembly. Many of these insertions are new members of existing gene families whose source genes are highly and widely expressed, and the majority have detectable hallmarks of processed gene retrocopy formation. We estimate the rate of novel gene retrocopy insertions in humans and chimps at roughly one new gene retrocopy insertion for every 6,000 individuals.
Conclusions: We find that gene retrocopy polymorphisms are a widespread phenomenon, present a multi-species analysis of these events, and provide a method for their ascertainment.
Figures
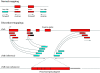
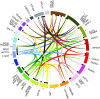
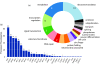
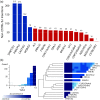
Similar articles
-
Retroposed copies of RET gene: a somatically acquired event in medullary thyroid carcinoma.BMC Med Genomics. 2019 Jul 9;12(1):104. doi: 10.1186/s12920-019-0552-1. BMC Med Genomics. 2019. PMID: 31288802 Free PMC article.
-
Somatic retrotransposition in human cancer revealed by whole-genome and exome sequencing.Genome Res. 2014 Jul;24(7):1053-63. doi: 10.1101/gr.163659.113. Epub 2014 May 13. Genome Res. 2014. PMID: 24823667 Free PMC article.
-
Comparative analysis of processed ribosomal protein pseudogenes in four mammalian genomes.Genome Biol. 2009;10(1):R2. doi: 10.1186/gb-2009-10-1-r2. Epub 2009 Jan 5. Genome Biol. 2009. PMID: 19123937 Free PMC article.
-
[Evolutionary recent insertions of mobile elements and their contribution to the structure of human genome].Zh Obshch Biol. 2012 Jan-Feb;73(1):3-20. Zh Obshch Biol. 2012. PMID: 22567964 Review. Russian.
-
Mammalian retroelements.Genome Res. 2002 Oct;12(10):1455-65. doi: 10.1101/gr.282402. Genome Res. 2002. PMID: 12368238 Review.
Cited by
-
svaRetro and svaNUMT: modular packages for annotating retrotransposed transcripts and nuclear integration of mitochondrial DNA in genome sequencing data.GigaByte. 2022 Oct 5;2022:gigabyte70. doi: 10.46471/gigabyte.70. eCollection 2022. GigaByte. 2022. PMID: 36824522 Free PMC article.
-
Heritable L1 Retrotransposition Events During Development: Understanding Their Origins: Examination of heritable, endogenous L1 retrotransposition in mice opens up exciting new questions and research directions.Bioessays. 2018 Jun;40(6):e1700189. doi: 10.1002/bies.201700189. Epub 2018 Apr 30. Bioessays. 2018. PMID: 29709066 Free PMC article.
-
Retroposed copies of RET gene: a somatically acquired event in medullary thyroid carcinoma.BMC Med Genomics. 2019 Jul 9;12(1):104. doi: 10.1186/s12920-019-0552-1. BMC Med Genomics. 2019. PMID: 31288802 Free PMC article.
-
Discovery of non-reference processed pseudogenes in the Swedish population.Front Genet. 2023 May 30;14:1176626. doi: 10.3389/fgene.2023.1176626. eCollection 2023. Front Genet. 2023. PMID: 37323659 Free PMC article.
-
Ancient segmentally duplicated LCORL retrocopies in equids.PLoS One. 2023 Jun 8;18(6):e0286861. doi: 10.1371/journal.pone.0286861. eCollection 2023. PLoS One. 2023. PMID: 37289743 Free PMC article.
References
-
- Karro JE, Yan Y, Zheng D, Zhang Z, Carriero N, Cayting P, Harrrison P, Gerstein M. Pseudogene.org: comprehensive database and comparison platform for pseudogene annotation. Nucleic Acids Research. 2007;35:D55–60. doi: 10.1093/nar/gkl851. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

