Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection
- PMID: 23441171
- PMCID: PMC3575416
- DOI: 10.1371/journal.pone.0056265
Multiple cationic amphiphiles induce a Niemann-Pick C phenotype and inhibit Ebola virus entry and infection
Erratum in
- PLoS One. 2013;8(10). doi:10.1371/annotation/76780c06-ac81-48a3-8cce-509da6858fe5
Abstract
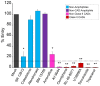
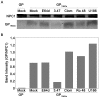
Ebola virus (EBOV) is an enveloped RNA virus that causes hemorrhagic fever in humans and non-human primates. Infection requires internalization from the cell surface and trafficking to a late endocytic compartment, where viral fusion occurs, providing a conduit for the viral genome to enter the cytoplasm and initiate replication. In a concurrent study, we identified clomiphene as a potent inhibitor of EBOV entry. Here, we screened eleven inhibitors that target the same biosynthetic pathway as clomiphene. From this screen we identified six compounds, including U18666A, that block EBOV infection (IC(50) 1.6 to 8.0 µM) at a late stage of entry. Intriguingly, all six are cationic amphiphiles that share additional chemical features. U18666A induces phenotypes, including cholesterol accumulation in endosomes, associated with defects in Niemann-Pick C1 protein (NPC1), a late endosomal and lysosomal protein required for EBOV entry. We tested and found that all six EBOV entry inhibitors from our screen induced cholesterol accumulation. We further showed that higher concentrations of cationic amphiphiles are required to inhibit EBOV entry into cells that overexpress NPC1 than parental cells, supporting the contention that they inhibit EBOV entry in an NPC1-dependent manner. A previously reported inhibitor, compound 3.47, inhibits EBOV entry by blocking binding of the EBOV glycoprotein to NPC1. None of the cationic amphiphiles tested had this effect. Hence, multiple cationic amphiphiles (including several FDA approved agents) inhibit EBOV entry in an NPC1-dependent fashion, but by a mechanism distinct from that of compound 3.47. Our findings suggest that there are minimally two ways of perturbing NPC1-dependent pathways that can block EBOV entry, increasing the attractiveness of NPC1 as an anti-filoviral therapeutic target.
Conflict of interest statement
Figures


Similar articles
-
Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection.Nature. 2011 Aug 24;477(7364):344-8. doi: 10.1038/nature10380. Nature. 2011. PMID: 21866101 Free PMC article.
-
Direct Visualization of Ebola Virus Fusion Triggering in the Endocytic Pathway.mBio. 2016 Feb 9;7(1):e01857-15. doi: 10.1128/mBio.01857-15. mBio. 2016. PMID: 26861015 Free PMC article.
-
Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step.J Virol. 2015 Mar;89(5):2931-43. doi: 10.1128/JVI.03398-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552710 Free PMC article.
-
Potential pharmacological strategies targeting the Niemann-Pick C1 receptor and Ebola virus glycoprotein interaction.Eur J Med Chem. 2021 Nov 5;223:113654. doi: 10.1016/j.ejmech.2021.113654. Epub 2021 Jun 19. Eur J Med Chem. 2021. PMID: 34175537 Review.
-
Filovirus entry: a novelty in the viral fusion world.Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review.
Cited by
-
Human Ebola virus infection in West Africa: a review of available therapeutic agents that target different steps of the life cycle of Ebola virus.Infect Dis Poverty. 2014 Nov 28;3:43. doi: 10.1186/2049-9957-3-43. eCollection 2014. Infect Dis Poverty. 2014. PMID: 25699183 Free PMC article.
-
Structural Basis of Pan-Ebolavirus Neutralization by an Antibody Targeting the Glycoprotein Fusion Loop.Cell Rep. 2018 Sep 4;24(10):2723-2732.e4. doi: 10.1016/j.celrep.2018.08.009. Cell Rep. 2018. PMID: 30184505 Free PMC article.
-
Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers.Biochim Biophys Acta Mol Cell Res. 2019 Jul;1866(7):1151-1161. doi: 10.1016/j.bbamcr.2018.10.022. Epub 2018 Nov 5. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30408544 Free PMC article.
-
Genomics and High-Consequence Infectious Diseases: A Scoping Review of Emerging Science and Potential Ethical Issues.Health Secur. 2019 Jan/Feb;17(1):62-68. doi: 10.1089/hs.2018.0108. Epub 2019 Feb 6. Health Secur. 2019. PMID: 30724614 Free PMC article.
-
Filovirus Antiviral Activity of Cationic Amphiphilic Drugs Is Associated with Lipophilicity and Ability To Induce Phospholipidosis.Antimicrob Agents Chemother. 2020 Jul 22;64(8):e00143-20. doi: 10.1128/AAC.00143-20. Print 2020 Jul 22. Antimicrob Agents Chemother. 2020. PMID: 32513799 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

