Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway
- PMID: 23411497
- PMCID: PMC3563200
Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway
Abstract
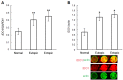
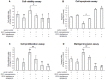
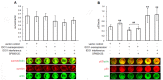
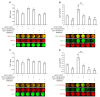
Evidence for an immunosuppressive function of indoleamine 2,3-dioxygenase (IDO) has been accumulating. However, the unusual distribution of IDO1 in gynecologic cancer cells suggests that modulating immunity may not its only function. To clarify the physiological importance of IDO1 in endometriosis, a tumor-like benign disease, we have investigated the potential mechanism by which IDO1 modulated endometrial stromal cells (ESCs) proliferation and invasion. ESCs were obtained from 16 control women (normal) and 14 patients with ovarian endometrioma, then the normal ESCs were treated with plasmid pEGFP-N1-IDO1 or SD11-IDO1 short hairpin RNA (shRNA) alone, or in combination with c-Jun N-terminal kinase (JNK) inhibitor (SP600125), and subjected to cell viability, proliferation, apoptosis assay and Matrigel invasion assay. IDO1 mRNA expression was evaluated by quantitative real-time reverse transcription-polymerase chain reaction (real-time PCR), and protein levels of IDO1, survivin, protein 53 (p53), matrix metalloproteinase (MMP)-2, MMP-9, tissue-inhibitor of metalloproteinase-1 (TIMP-1) and cyclooxygenase-2 (COX-2) in IDO1-overexpressing and IDO1-deficiency ESCs were analyzed by in-cell Western. We found that IDO1 expression was higher in endometriosis-derived eutopic and ectopic ESCs, compared with endometriosis-free normal ESCs. As a result, IDO1-overexpression in ESCs was markedly linked to reduction of apoptosis and p53 expression, and upregulation of survival, proliferation, invasion, as well as expression of MMP-9, COX-2 expression, rather than expression of survivin, MMP-2 and TIMP-1. Reversely, JNK blockage could abrogate these alterations of ESCs in IDO1-overexpressing milieu, suggesting that JNK signaling pathway was indispensable for ESCs survival, proliferation and invasion enhanced by IDO1, which may contribute to the pathophysiology of endometriosis.
Keywords: 3-dioxygenase; Endometriosis; endometrial stromal cells; indoleamine 2.
Figures

Similar articles
-
Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells.Mol Hum Reprod. 2012 Oct;18(10):467-76. doi: 10.1093/molehr/gas021. Epub 2012 May 24. Mol Hum Reprod. 2012. PMID: 22638210
-
Indoleamine 2,3-dioxygenase-1 (IDO1) in human endometrial stromal cells induces macrophage tolerance through interleukin-33 in the progression of endometriosis.Int J Clin Exp Pathol. 2014 May 15;7(6):2743-57. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25031694 Free PMC article.
-
Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway.Fertil Steril. 2012 Apr;97(4):919-29. doi: 10.1016/j.fertnstert.2011.12.049. Epub 2012 Jan 20. Fertil Steril. 2012. PMID: 22265030
-
Immunosuppressive macrophages induced by IDO1 promote the growth of endometrial stromal cells in endometriosis.Mol Med Rep. 2017 Apr;15(4):2255-2260. doi: 10.3892/mmr.2017.6242. Epub 2017 Feb 22. Mol Med Rep. 2017. PMID: 28260094
-
An overview on the methods of determining the activity of Indoleamine 2, 3-Dioxygenase 1.J Drug Target. 2019 Aug;27(7):724-731. doi: 10.1080/1061186X.2018.1523416. Epub 2018 Dec 5. J Drug Target. 2019. PMID: 30215536 Review.
Cited by
-
H2S promotes proliferation of endometrial stromal cells via activating the NF-κB pathway in endometriosis.Am J Transl Res. 2018 Dec 15;10(12):4247-4257. eCollection 2018. Am J Transl Res. 2018. PMID: 30662667 Free PMC article.
-
Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms.Am J Ther. 2018 Mar/Apr;25(2):e194-e201. doi: 10.1097/MJT.0000000000000584. Am J Ther. 2018. PMID: 28901958 Free PMC article. Clinical Trial.
-
Endometrial biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 Apr 20;4(4):CD012165. doi: 10.1002/14651858.CD012165. Cochrane Database Syst Rev. 2016. PMID: 27094925 Free PMC article. Review.
-
Maternal embryonic leucine zipper kinase: key kinase for stem cell phenotype in glioma and other cancers.Mol Cancer Ther. 2014 Jun;13(6):1393-8. doi: 10.1158/1535-7163.MCT-13-0764. Epub 2014 May 2. Mol Cancer Ther. 2014. PMID: 24795222 Free PMC article. Review.
-
Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16ink4a and β-Galactosidase in Stromal Cells.Int J Mol Sci. 2023 Jan 4;24(2):914. doi: 10.3390/ijms24020914. Int J Mol Sci. 2023. PMID: 36674426 Free PMC article.
References
-
- Strathy JH, Molgaard CA, Coulam CB, Melton LJ 3rd. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril. 1982;38:667–72. - PubMed
-
- Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, Ueki M. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–9. - PubMed
-
- Palafox D, Llorente L, Alberú J, Torres-Machorro A, Camorlinga N, Rodríguez C, Nyström J. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando) 2010;24:160–5. - PubMed
-
- Entrican G, Wattegedera S, Rocchi M, Wheelhouse N. Pregnancy, indoleamine 2,3-dioxygenase (IDO) and chlamydial abortion: an unresolved paradox. Vet Microbiol. 2009;135:98–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
