The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737
- PMID: 23341456
- PMCID: PMC3591608
- DOI: 10.1074/jbc.M112.414177
The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737
Abstract
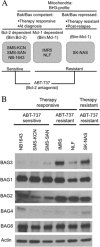
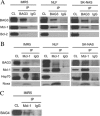
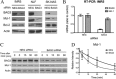
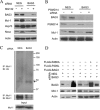
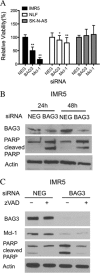
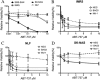
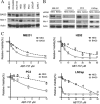
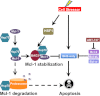
Members of the Bcl-2 family of proteins are important inhibitors of apoptosis in human cancer and are targets for novel anticancer agents such as the Bcl-2 antagonists, ABT-263 (Navitoclax), and its analog ABT-737. Unlike Bcl-2, Mcl-1 is not antagonized by ABT-263 or ABT-737 and is considered to be a major factor in resistance. Also, Mcl-1 exhibits differential regulation when compared with other Bcl-2 family members and is a target for anticancer drug discovery. Here, we demonstrate that BAG3, an Hsp70 co-chaperone, protects Mcl-1 from proteasomal degradation, thereby promoting its antiapoptotic activity. Using neuroblastoma cell lines, with a defined Bcl-2 family dependence, we found that BAG3 expression correlated with Mcl-1 dependence and ABT-737 resistance. RNA silencing of BAG3 led to a marked reduction in Mcl-1 protein levels and overcame ABT-737 resistance in Mcl-1-dependent cells. In ABT-737-resistant cells, Mcl-1 co-immunoprecipitated with BAG3, and loss of Mcl-1 after BAG3 silencing was prevented by proteasome inhibition. BAG3 and Mcl-1 were co-expressed in a panel of diverse cancer cell lines resistant to ABT-737. Silencing BAG3 reduced Mcl-1 protein levels and overcame ABT-737 resistance in several of the cell lines, including triple-negative breast cancer (MDA-MB231) and androgen receptor-negative prostate cancer (PC3) cells. These studies identify BAG3-mediated Mcl-1 stabilization as a potential target for cancer drug discovery.
Figures
Similar articles
-
Proteasomal degradation of Mcl-1 by maritoclax induces apoptosis and enhances the efficacy of ABT-737 in melanoma cells.PLoS One. 2013 Nov 4;8(11):e78570. doi: 10.1371/journal.pone.0078570. eCollection 2013. PLoS One. 2013. PMID: 24223823 Free PMC article.
-
'Seed' analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737.Oncogene. 2007 Jun 7;26(27):3972-9. doi: 10.1038/sj.onc.1210166. Epub 2006 Dec 18. Oncogene. 2007. PMID: 17173063
-
Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1.Blood. 2010 Apr 22;115(16):3304-13. doi: 10.1182/blood-2009-07-233304. Epub 2010 Mar 2. Blood. 2010. PMID: 20197552 Free PMC article.
-
Targeting multiple arms of the apoptotic regulatory machinery.Cancer Res. 2007 Apr 1;67(7):2908-11. doi: 10.1158/0008-5472.CAN-07-0082. Cancer Res. 2007. PMID: 17409392 Review.
-
BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions.Cancer Lett. 2013 May 28;332(2):202-5. doi: 10.1016/j.canlet.2011.12.021. Epub 2012 Jan 8. Cancer Lett. 2013. PMID: 22230093 Free PMC article. Review.
Cited by
-
miR-22 and miR-29a Are Members of the Androgen Receptor Cistrome Modulating LAMC1 and Mcl-1 in Prostate Cancer.Mol Endocrinol. 2015 Jul;29(7):1037-54. doi: 10.1210/me.2014-1358. Epub 2015 Jun 8. Mol Endocrinol. 2015. PMID: 26052614 Free PMC article.
-
Targeting Apoptosis in Cancer.Curr Oncol Rep. 2022 Mar;24(3):273-284. doi: 10.1007/s11912-022-01199-y. Epub 2022 Feb 3. Curr Oncol Rep. 2022. PMID: 35113355 Review.
-
Optimal targeting of BCL-family proteins in head and neck squamous cell carcinoma requires inhibition of both BCL-xL and MCL-1.Oncotarget. 2019 Jan 11;10(4):494-510. doi: 10.18632/oncotarget.26563. eCollection 2019 Jan 11. Oncotarget. 2019. PMID: 30728900 Free PMC article.
-
Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death.Cell Death Dis. 2013 Oct 3;4(10):e834. doi: 10.1038/cddis.2013.360. Cell Death Dis. 2013. PMID: 24091677 Free PMC article.
-
TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo.Oncotarget. 2015 Nov 3;6(34):36456-71. doi: 10.18632/oncotarget.5505. Oncotarget. 2015. PMID: 26474387 Free PMC article.
References
-
- Green D. R., Evan G. I. (2002) A matter of life and death. Cancer Cell 1, 19–30 - PubMed
-
- Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 - PubMed
-
- Reed J. C., Cuddy M., Slabiak T., Croce C. M., Nowell P. C. (1988) Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature 336, 259–261 - PubMed
-
- Yip K. W., Reed J. C. (2008) Bcl-2 family proteins and cancer. Oncogene 27, 6398–6406 - PubMed
-
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

