Essentials of Th17 cell commitment and plasticity
- PMID: 23325835
- PMCID: PMC3612853
- DOI: 10.1182/blood-2012-09-378653
Essentials of Th17 cell commitment and plasticity
Abstract
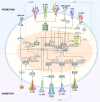
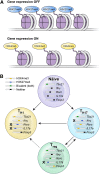
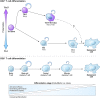
CD4(+) T helper (Th) cells exist in a variety of epigenetic states that determine their function, phenotype, and capacity for persistence. These polarization states include Th1, Th2, Th17, and Foxp3(+) T regulatory cells, as well as the more recently described T follicular helper, Th9, and Th22 cells. Th17 cells express the master transcriptional regulator retinoic acid-related orphan receptor γ thymus and produce canonical interleukin (IL)-17A and IL-17F cytokines. Th17 cells display a great degree of context-dependent plasticity, as they are capable of acquiring functional characteristics of Th1 cells. This late plasticity may contribute to the protection against microbes, plays a role in the development of autoimmunity, and is necessary for antitumor activity of Th17 cells in adoptive cell transfer therapy models. Moreover, plasticity of this subset is associated with higher in vivo survival and self-renewal capacity and less senescence than Th1 polarized cells, which have less plasticity and more phenotypic stability. New findings indicate that subset polarization of CD4(+) T cells not only induces characteristic patterns of surface markers and cytokine production but also has a maturational aspect that affects a cell's ability to survive, respond to secondary stimulation, and form long-term immune memory.
Figures
Similar articles
-
A cellular and molecular view of T helper 17 cell plasticity in autoimmunity.J Autoimmun. 2018 Feb;87:1-15. doi: 10.1016/j.jaut.2017.12.007. Epub 2017 Dec 22. J Autoimmun. 2018. PMID: 29275836 Review.
-
Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets.Stem Cell Rev Rep. 2015 Jun;11(3):394-407. doi: 10.1007/s12015-014-9558-4. Stem Cell Rev Rep. 2015. PMID: 25348066 Free PMC article.
-
Role and plasticity of Th1 and Th17 responses in immunity to Staphylococcus aureus.Hum Vaccin Immunother. 2019;15(12):2980-2992. doi: 10.1080/21645515.2019.1613126. Epub 2019 Oct 31. Hum Vaccin Immunother. 2019. PMID: 31149870 Free PMC article. Clinical Trial.
-
TLR-stimulated CD34 stem cell-derived human skin-like and monocyte-derived dendritic cells fail to induce Th17 polarization of naive T cells but do stimulate Th1 and Th17 memory responses.J Immunol. 2009 Aug 15;183(4):2242-51. doi: 10.4049/jimmunol.0900474. Epub 2009 Jul 22. J Immunol. 2009. PMID: 19625644
-
Cytokine-regulated Th17 plasticity in human health and diseases.Immunology. 2021 May;163(1):3-18. doi: 10.1111/imm.13280. Epub 2020 Nov 6. Immunology. 2021. PMID: 33064842 Free PMC article. Review.
Cited by
-
Viral immune surveillance: Toward a TH17/TH9 gate to the central nervous system.Bioinformation. 2015 Jan 30;11(1):47-54. doi: 10.6026/97320630011047. eCollection 2015. Bioinformation. 2015. PMID: 25780281 Free PMC article.
-
Regulation of pulmonary graft-versus-host disease by IL-26+CD26+CD4 T lymphocytes.J Immunol. 2015 Apr 15;194(8):3697-712. doi: 10.4049/jimmunol.1402785. Epub 2015 Mar 18. J Immunol. 2015. PMID: 25786689 Free PMC article.
-
Recent advances and research progress regarding monoclonal antibodies for chronic graft-versus-host disease.Heliyon. 2024 Sep 25;10(19):e38460. doi: 10.1016/j.heliyon.2024.e38460. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39403509 Free PMC article. Review.
-
Gut Microbiome Homeostasis and the CD4 T- Follicular Helper Cell IgA Axis in Human Immunodeficiency Virus Infection.Front Immunol. 2021 Mar 19;12:657679. doi: 10.3389/fimmu.2021.657679. eCollection 2021. Front Immunol. 2021. PMID: 33815419 Free PMC article. Review.
-
Bona Fide Th17 Cells without Th1 Functional Plasticity Protect against Influenza.J Immunol. 2022 Apr 15;208(8):1998-2007. doi: 10.4049/jimmunol.2100801. Epub 2022 Mar 25. J Immunol. 2022. PMID: 35338093 Free PMC article.
References
-
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

