Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells
- PMID: 23301832
- PMCID: PMC3560853
- DOI: 10.1111/jcmm.12004
Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells
Abstract
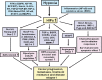
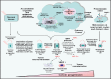
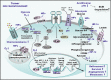
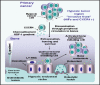
Accumulating lines of experimental evidence have revealed that hypoxia-inducible factors, HIF-1α and HIF-2α, are key regulators of the adaptation of cancer- and metastasis-initiating cells and their differentiated progenies to oxygen and nutrient deprivation during cancer progression under normoxic and hypoxic conditions. Particularly, the sustained stimulation of epidermal growth factor receptor (EGFR), insulin-like growth factor-1 receptor (IGF-1R), stem cell factor (SCF) receptor KIT, transforming growth factor-β receptors (TGF-βRs) and Notch and their downstream signalling elements such as phosphatidylinositol 3'-kinase (PI3K)/Akt/molecular target of rapamycin (mTOR) may lead to an enhanced activity of HIFs. Moreover, the up-regulation of HIFs in cancer cells may also occur in the hypoxic intratumoral regions formed within primary and secondary neoplasms as well as in leukaemic cells and metastatic prostate and breast cancer cells homing in the hypoxic endosteal niche of bone marrow. The activated HIFs may induce the expression of numerous gene products such as induced pluripotency-associated transcription factors (Oct-3/4, Nanog and Sox-2), glycolysis- and epithelial-mesenchymal transition (EMT) programme-associated molecules, including CXC chemokine receptor 4 (CXCR4), snail and twist, microRNAs and angiogenic factors such as vascular endothelial growth factor (VEGF). These gene products in turn can play critical roles for high self-renewal ability, survival, altered energy metabolism, invasion and metastases of cancer cells, angiogenic switch and treatment resistance. Consequently, the targeting of HIF signalling network and altered metabolic pathways represents new promising strategies to eradicate the total mass of cancer cells and improve the efficacy of current therapies against aggressive and metastatic cancers and prevent disease relapse.
© 2012 The Authors. Published by Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures
Similar articles
-
Altered gene products involved in the malignant reprogramming of cancer stem/progenitor cells and multitargeted therapies.Mol Aspects Med. 2014 Oct;39:3-32. doi: 10.1016/j.mam.2013.08.001. Epub 2013 Aug 29. Mol Aspects Med. 2014. PMID: 23994756 Free PMC article. Review.
-
Frequent gene products and molecular pathways altered in prostate cancer- and metastasis-initiating cells and their progenies and novel promising multitargeted therapies.Mol Med. 2011 Sep-Oct;17(9-10):949-64. doi: 10.2119/molmed.2011.00115. Epub 2011 May 20. Mol Med. 2011. PMID: 21607288 Free PMC article. Review.
-
ITGA6 is directly regulated by hypoxia-inducible factors and enriches for cancer stem cell activity and invasion in metastatic breast cancer models.Mol Cancer. 2016 Mar 22;15:26. doi: 10.1186/s12943-016-0510-x. Mol Cancer. 2016. PMID: 27001172 Free PMC article.
-
CD44 Interacts with HIF-2α to Modulate the Hypoxic Phenotype of Perinecrotic and Perivascular Glioma Cells.Cell Rep. 2017 Aug 15;20(7):1641-1653. doi: 10.1016/j.celrep.2017.07.049. Cell Rep. 2017. PMID: 28813675
-
HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways.J Exp Clin Cancer Res. 2018 Oct 19;37(1):256. doi: 10.1186/s13046-018-0925-x. J Exp Clin Cancer Res. 2018. PMID: 30340507 Free PMC article.
Cited by
-
Pregnancy and Cancer: Cellular Biology and Mechanisms Affecting the Placenta.Cancers (Basel). 2021 Apr 1;13(7):1667. doi: 10.3390/cancers13071667. Cancers (Basel). 2021. PMID: 33916290 Free PMC article. Review.
-
Potential role of hypoxia in early stages of Hodgkin lymphoma pathogenesis.Haematologica. 2015 Oct;100(10):1320-6. doi: 10.3324/haematol.2015.127498. Epub 2015 Jul 9. Haematologica. 2015. PMID: 26160878 Free PMC article.
-
Balancing STAT Activity as a Therapeutic Strategy.Cancers (Basel). 2019 Nov 3;11(11):1716. doi: 10.3390/cancers11111716. Cancers (Basel). 2019. PMID: 31684144 Free PMC article. Review.
-
The roles of HLH transcription factors in epithelial mesenchymal transition and multiple molecular mechanisms.Clin Exp Metastasis. 2014 Mar;31(3):367-77. doi: 10.1007/s10585-013-9621-6. Epub 2013 Oct 26. Clin Exp Metastasis. 2014. PMID: 24158354 Review.
-
Cancer stem cells and tumor metastasis (Review).Int J Oncol. 2014 Jun;44(6):1806-12. doi: 10.3892/ijo.2014.2362. Epub 2014 Apr 2. Int J Oncol. 2014. PMID: 24691919 Free PMC article. Review.
References
-
- Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1 alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. - PubMed
-
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

