Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway
- PMID: 23291594
- PMCID: PMC3663146
- DOI: 10.1016/j.freeradbiomed.2012.12.019
Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway
Abstract
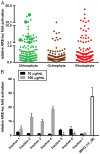
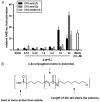
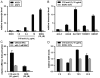
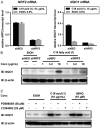
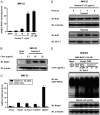
Increased amounts of reactive oxygen species (ROS) have been implicated in many pathological conditions, including cancer. The major machinery that the cell employs to neutralize excess ROS is through the activation of the antioxidant-response element (ARE) that controls the activation of many phase II detoxification enzymes. The transcription factor that recognizes the ARE, Nrf2, can be activated by a variety of small molecules, most of which contain an α,β-unsaturated carbonyl system. In the pursuit of chemopreventive agents from marine organisms, we built, fractionated, and screened a library of 30 field-collected eukaryotic algae from Florida. An edible green alga, Ulva lactuca, yielded multiple active fractions by ARE-luciferase reporter assay. We isolated three monounsaturated fatty acid (MUFA) derivatives as active components, including a new keto-type C18 fatty acid (1), the corresponding shorter chain C16 acid (2), and an amide derivative (3) of the C18 acid. Their chemical structures were elucidated by NMR and mass spectrometry. All three contain the conjugated enone motif between C7 and C9, which is thought to be responsible for the ARE activity. Subsequent biological studies focused on 1, the most active and abundant ARE activator isolated. C18 acid 1 induced the expression of ARE-regulated cytoprotective genes, including NAD(P)H:quinone oxidoreductase 1, heme oxygenase 1, thioredoxin reductase 1, both subunits of the glutamate-cysteine ligase (catalytic subunit and modifier subunit), and the cystine/glutamate exchange transporter, in IMR-32 human neuroblastoma cells. Its cellular activity requires the presence of Nrf2 and PI3K function, based on RNA interference and pharmacological inhibitor studies, respectively. Treatment with 1 led only to Nrf2 activation, and not the increase in production of NRF2 mRNA. To test its ARE activity and cytoprotective potential in vivo, we treated mice with a single dose of a U. lactuca fraction that was enriched with 1, which showed ARE-activating effects similar to those observed in vitro. This could be owing to this fraction's ability to stabilize Nrf2 through inhibition of Keap1-mediated Nrf2 ubiquitination and the subsequent accumulation and nuclear translocation of Nrf2. The induction of many ARE-driven antioxidant genes in vivo and most prominently in the heart agreed with the commonly recognized cardioprotective properties of MUFAs. A significant increase in Nqo1 transcript levels was also found in other mouse tissues such as the brain, lung, and stomach. Collectively, this study provides new insight into why consumption of dietary seaweed may have health benefits, and the identified compounds add to the list of chemopreventive dietary unsaturated fatty acids.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

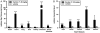
Similar articles
-
Cultivated sea lettuce is a multiorgan protector from oxidative and inflammatory stress by enhancing the endogenous antioxidant defense system.Cancer Prev Res (Phila). 2013 Sep;6(9):989-99. doi: 10.1158/1940-6207.CAPR-13-0014. Cancer Prev Res (Phila). 2013. PMID: 24005795 Free PMC article.
-
Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway.Cell Signal. 2014 Mar;26(3):512-20. doi: 10.1016/j.cellsig.2013.11.029. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308969
-
PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells.Cell Biol Toxicol. 2013 Jun;29(3):143-57. doi: 10.1007/s10565-013-9242-5. Epub 2013 Mar 24. Cell Biol Toxicol. 2013. PMID: 23525690
-
Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals.Planta Med. 2008 Oct;74(13):1526-39. doi: 10.1055/s-0028-1088302. Epub 2008 Oct 20. Planta Med. 2008. PMID: 18937164 Review.
-
Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging.Int J Biochem Cell Biol. 2012 Aug;44(8):1315-20. doi: 10.1016/j.biocel.2012.04.021. Epub 2012 May 7. Int J Biochem Cell Biol. 2012. PMID: 22575091 Review.
Cited by
-
Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis.Reprod Biol Endocrinol. 2013 Mar 21;11:23. doi: 10.1186/1477-7827-11-23. Reprod Biol Endocrinol. 2013. PMID: 23514035 Free PMC article.
-
A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants.Int J Mol Sci. 2020 Oct 21;21(20):7774. doi: 10.3390/ijms21207774. Int J Mol Sci. 2020. PMID: 33096625 Free PMC article. Review.
-
Green seaweeds fatty acids and heterocyclic derivatives against cancer: Opinion on future nutraceutical application.Front Oncol. 2023 Feb 14;13:1145919. doi: 10.3389/fonc.2023.1145919. eCollection 2023. Front Oncol. 2023. PMID: 36865809 Free PMC article. No abstract available.
-
Effects of dietary Enteromorpha powder supplementation on productive performance, egg quality, and antioxidant performance during the late laying period in Zi geese.Poult Sci. 2020 Feb;99(2):1062-1068. doi: 10.1016/j.psj.2019.10.003. Epub 2019 Nov 21. Poult Sci. 2020. PMID: 32029142 Free PMC article.
-
The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders.Clin Pharmacol. 2014 Feb 3;6:19-34. doi: 10.2147/CPAA.S35078. eCollection 2014. Clin Pharmacol. 2014. PMID: 24520207 Free PMC article. Review.
References
-
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. - PubMed
-
- Zhang DD. Mechanistic studies of the Nrf2–Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. - PubMed
-
- Kobayashi M, Yamamoto M. Nrf2–Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. - PubMed
-
- Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

