Transforming growth factor-β directly induces p53-up-regulated modulator of apoptosis (PUMA) during the rapid induction of apoptosis in myc-driven B-cell lymphomas
- PMID: 23243310
- PMCID: PMC3576124
- DOI: 10.1074/jbc.M112.410274
Transforming growth factor-β directly induces p53-up-regulated modulator of apoptosis (PUMA) during the rapid induction of apoptosis in myc-driven B-cell lymphomas
Abstract
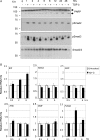
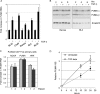
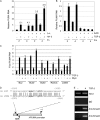
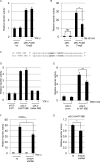
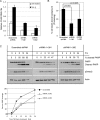
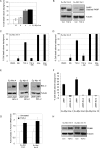
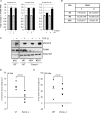
c-Myc transformed human Burkitt's lymphoma (BL) cells are highly sensitive to TGF-β-induced apoptosis. Previously we demonstrated that TGF-β-mediated cell death in BL cells is regulated via the mitochondrial intrinsic apoptosis pathway, which is dependent on the activation of BAX and/or BAK. TGF-β directly induces transcription of the BH3-only protein BIK and represses expression of the pro-survival factor BCL-X(L) but has no effect on the direct BAX/BAK "activators" BIM or BID (tBID). Here we show that TGF-β induces the BH3-only activator PUMA to aid induction of the intrinsic cell death pathway. TGF-β also induced PUMA in normal germinal center CD77-positive centroblasts isolated from human tonsil tissue. PUMA was a direct TGF-β target gene in B-cells, and we identify a putative Smad-binding region within the human PUMA promoter that recruits Smad3 and Smad4 in cells in response to TGF-β signaling. Constitutive activity of the isolated Smad-binding region in luciferase reporter assays was dependent on Smad consensus sequences and was partially dependent on endogenous TGF-β signaling and Smad4. Knockdown of PUMA in BL cells using lentiviral shRNA resulted in slower kinetics of the TGF-β-mediated apoptotic response. Analysis of Eμ-Myc cell lines demonstrated that c-myc-driven murine lymphomas are also sensitive to TGF-β-mediated apoptosis. Moreover, Puma(-/-) Eμ-Myc lines demonstrated significantly delayed kinetics of the apoptotic response when compared with wild type lymphomas. TGF-β therefore induces a polygenic response in Myc-driven lymphomas involving transcription of PUMA, which is necessary for the rapid induction of cell death.
Figures
Similar articles
-
Combined loss of PUMA and p21 accelerates c-MYC-driven lymphoma development considerably less than loss of one allele of p53.Oncogene. 2016 Jul 21;35(29):3866-71. doi: 10.1038/onc.2015.457. Epub 2015 Dec 7. Oncogene. 2016. PMID: 26640149
-
TGF-beta induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-XL.Cell Death Differ. 2009 Apr;16(4):593-602. doi: 10.1038/cdd.2008.183. Epub 2009 Jan 9. Cell Death Differ. 2009. PMID: 19136942 Free PMC article.
-
Bax/Bak activation in the absence of Bid, Bim, Puma, and p53.Cell Death Dis. 2016 Jun 16;7(6):e2266. doi: 10.1038/cddis.2016.167. Cell Death Dis. 2016. PMID: 27310874 Free PMC article.
-
PUMA, a critical mediator of cell death--one decade on from its discovery.Cell Mol Biol Lett. 2012 Dec;17(4):646-69. doi: 10.2478/s11658-012-0032-5. Epub 2012 Sep 20. Cell Mol Biol Lett. 2012. PMID: 23001513 Free PMC article. Review.
-
The ARTS of p53-dependent mitochondrial apoptosis.J Mol Cell Biol. 2023 Mar 29;14(10):mjac074. doi: 10.1093/jmcb/mjac074. J Mol Cell Biol. 2023. PMID: 36565718 Free PMC article. Review.
Cited by
-
SF3B1 deficiency impairs human erythropoiesis via activation of p53 pathway: implications for understanding of ineffective erythropoiesis in MDS.J Hematol Oncol. 2018 Feb 12;11(1):19. doi: 10.1186/s13045-018-0558-8. J Hematol Oncol. 2018. PMID: 29433555 Free PMC article.
-
RGS5-TGFβ-Smad2/3 axis switches pro- to anti-apoptotic signaling in tumor-residing pericytes, assisting tumor growth.Cell Death Differ. 2021 Nov;28(11):3052-3076. doi: 10.1038/s41418-021-00801-3. Epub 2021 May 19. Cell Death Differ. 2021. PMID: 34012071 Free PMC article.
-
Combined BCL-2 and PI3K/AKT Pathway Inhibition in KMT2A-Rearranged Acute B-Lymphoblastic Leukemia Cells.Int J Mol Sci. 2023 Jan 10;24(2):1359. doi: 10.3390/ijms24021359. Int J Mol Sci. 2023. PMID: 36674872 Free PMC article.
-
Noxa and Puma genes regulated by hTERT promoter can mitigate growth and induce apoptosis in hepatocellular carcinoma mouse model.J Cancer. 2022 Mar 28;13(6):2001-2013. doi: 10.7150/jca.70282. eCollection 2022. J Cancer. 2022. PMID: 35399714 Free PMC article.
-
TGF-β3 Inhibits Antibody Production by Human B Cells.PLoS One. 2017 Jan 4;12(1):e0169646. doi: 10.1371/journal.pone.0169646. eCollection 2017. PLoS One. 2017. PMID: 28052118 Free PMC article.
References
-
- Li M. O., Wan Y. Y., Sanjabi S., Robertson A. K., Flavell R. A. (2006) Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 - PubMed
-
- Inman G. J., Allday M. J. (2000) Apoptosis induced by TGF-β1 in Burkitt's lymphoma cells is caspase 8 dependent but is death receptor independent. J. Immunol. 165, 2500–2510 - PubMed
-
- Shi Y., Massagué J. (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 - PubMed
-
- Derynck R., Zhang Y. E. (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

