Hsp90 inhibitors are efficacious against Kaposi Sarcoma by enhancing the degradation of the essential viral gene LANA, of the viral co-receptor EphA2 as well as other client proteins
- PMID: 23209418
- PMCID: PMC3510261
- DOI: 10.1371/journal.ppat.1003048
Hsp90 inhibitors are efficacious against Kaposi Sarcoma by enhancing the degradation of the essential viral gene LANA, of the viral co-receptor EphA2 as well as other client proteins
Abstract
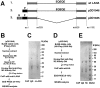
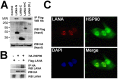
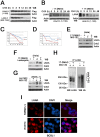
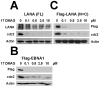
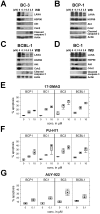
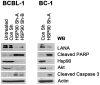
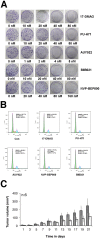
Heat-shock protein 90 (Hsp90) inhibitors exhibit activity against human cancers. We evaluated a series of new, oral bioavailable, chemically diverse Hsp90 inhibitors (PU-H71, AUY922, BIIB021, NVP-BEP800) against Kaposi sarcoma (KS). All Hsp90 inhibitors exhibited nanomolar EC(50) in culture and AUY922 reduced tumor burden in a xenograft model of KS. KS is associated with KS-associated herpesvirus (KSHV). We identified the viral latency associated nuclear antigen (LANA) as a novel client protein of Hsp90 and demonstrate that the Hsp90 inhibitors diminish the level of LANA through proteasomal degradation. These Hsp90 inhibitors also downregulated EphA2 and ephrin-B2 protein levels. LANA is essential for viral maintenance and EphA2 has recently been shown to facilitate KSHV infection; which in turn feeds latent persistence. Further, both molecules are required for KS tumor formation and both were downregulated in response to Hsp90 inhibitors. This provides a rationale for clinical testing of Hsp90 inhibitors in KSHV-associated cancers and in the eradication of latent KSHV reservoirs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Kaposi's sarcoma-associated herpesvirus-encoded LANA interacts with host KAP1 to facilitate establishment of viral latency.J Virol. 2014 Jul;88(13):7331-44. doi: 10.1128/JVI.00596-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741090 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded LANA contributes to viral latent replication by activating phosphorylation of survivin.J Virol. 2014 Apr;88(8):4204-17. doi: 10.1128/JVI.03855-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478433 Free PMC article.
-
Activated Nrf2 Interacts with Kaposi's Sarcoma-Associated Herpesvirus Latency Protein LANA-1 and Host Protein KAP1 To Mediate Global Lytic Gene Repression.J Virol. 2015 Aug;89(15):7874-92. doi: 10.1128/JVI.00895-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995248 Free PMC article.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
-
Viral latent proteins as targets for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma.Curr Drug Targets Infect Disord. 2003 Jun;3(2):129-35. doi: 10.2174/1568005033481150. Curr Drug Targets Infect Disord. 2003. PMID: 12769790 Review.
Cited by
-
Celastrol increases glucocerebrosidase activity in Gaucher disease by modulating molecular chaperones.Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):249-54. doi: 10.1073/pnas.1321341111. Epub 2013 Dec 18. Proc Natl Acad Sci U S A. 2014. PMID: 24351928 Free PMC article.
-
Hsp90 inhibitor 17-DMAG decreases expression of conserved herpesvirus protein kinases and reduces virus production in Epstein-Barr virus-infected cells.J Virol. 2013 Sep;87(18):10126-38. doi: 10.1128/JVI.01671-13. Epub 2013 Jul 10. J Virol. 2013. PMID: 23843639 Free PMC article.
-
How do viruses trick B cells into becoming lymphomas?Curr Opin Hematol. 2014 Jul;21(4):358-68. doi: 10.1097/MOH.0000000000000060. Curr Opin Hematol. 2014. PMID: 24886824 Free PMC article. Review.
-
Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies.PLoS Pathog. 2013;9(7):e1003484. doi: 10.1371/journal.ppat.1003484. Epub 2013 Jul 18. PLoS Pathog. 2013. PMID: 23874201 Free PMC article. Clinical Trial.
-
HSP90 protects the human T-cell leukemia virus type 1 (HTLV-1) tax oncoprotein from proteasomal degradation to support NF-κB activation and HTLV-1 replication.J Virol. 2013 Dec;87(24):13640-54. doi: 10.1128/JVI.02006-13. Epub 2013 Oct 9. J Virol. 2013. PMID: 24109220 Free PMC article.
References
-
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, et al. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120: 715–727. - PubMed
-
- Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11: 515–528. - PubMed
-
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR (2006) Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci 31: 164–172. - PubMed
-
- Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5: 761–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

