Applications of minimal physiologically-based pharmacokinetic models
- PMID: 23179857
- PMCID: PMC3539784
- DOI: 10.1007/s10928-012-9280-2
Applications of minimal physiologically-based pharmacokinetic models
Abstract
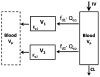
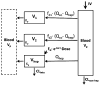
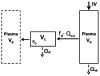
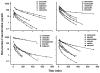
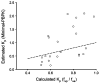
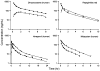
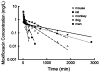
Conventional mammillary models are frequently used for pharmacokinetic (PK) analysis when only blood or plasma data are available. Such models depend on the quality of the drug disposition data and have vague biological features. An alternative minimal-physiologically-based PK (minimal-PBPK) modeling approach is proposed which inherits and lumps major physiologic attributes from whole-body PBPK models. The body and model are represented as actual blood and tissue (usually total body weight) volumes, fractions (f ( d )) of cardiac output with Fick's Law of Perfusion, tissue/blood partitioning (K ( p )), and systemic or intrinsic clearance. Analyzing only blood or plasma concentrations versus time, the minimal-PBPK models parsimoniously generate physiologically-relevant PK parameters which are more easily interpreted than those from mammillary models. The minimal-PBPK models were applied to four types of therapeutic agents and conditions. The models well captured the human PK profiles of 22 selected beta-lactam antibiotics allowing comparison of fitted and calculated K ( p ) values. Adding a classical hepatic compartment with hepatic blood flow allowed joint fitting of oral and intravenous (IV) data for four hepatic elimination drugs (dihydrocodeine, verapamil, repaglinide, midazolam) providing separate estimates of hepatic intrinsic clearance, non-hepatic clearance, and pre-hepatic bioavailability. The basic model was integrated with allometric scaling principles to simultaneously describe moxifloxacin PK in five species with common K ( p ) and f ( d ) values. A basic model assigning clearance to the tissue compartment well characterized plasma concentrations of six monoclonal antibodies in human subjects, providing good concordance of predictions with expected tissue kinetics. The proposed minimal-PBPK modeling approach offers an alternative and more rational basis for assessing PK than compartmental models.
Figures
Similar articles
-
Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies.J Pharmacokinet Pharmacodyn. 2013 Oct;40(5):597-607. doi: 10.1007/s10928-013-9332-2. Epub 2013 Aug 31. J Pharmacokinet Pharmacodyn. 2013. PMID: 23996115 Free PMC article.
-
Utility of Minimal Physiologically Based Pharmacokinetic Models for Assessing Fractional Distribution, Oral Absorption, and Series-Compartment Models of Hepatic Clearance.Drug Metab Dispos. 2023 Oct;51(10):1403-1418. doi: 10.1124/dmd.123.001403. Epub 2023 Jul 17. Drug Metab Dispos. 2023. PMID: 37460222 Free PMC article.
-
Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human.J Pharmacokinet Pharmacodyn. 2012 Feb;39(1):67-86. doi: 10.1007/s10928-011-9232-2. Epub 2011 Dec 6. J Pharmacokinet Pharmacodyn. 2012. PMID: 22143261
-
Physiologically-based pharmacokinetic modeling for absorption, transport, metabolism and excretion.J Pharmacokinet Pharmacodyn. 2010 Dec;37(6):591-615. doi: 10.1007/s10928-010-9185-x. Epub 2010 Dec 14. J Pharmacokinet Pharmacodyn. 2010. PMID: 21153869 Review.
-
Development of physiologically based pharmacokinetic and physiologically based pharmacodynamic models for applications in toxicology and risk assessment.Toxicol Lett. 1995 Sep;79(1-3):35-44. doi: 10.1016/0378-4274(95)03355-o. Toxicol Lett. 1995. PMID: 7570672 Review.
Cited by
-
Exploring the Impact of Pharmacological Target-Mediated Low Plasma Exposure in Lead Compound Selection in Drug Discovery - A Modeling Approach.AAPS J. 2024 Oct 28;26(6):112. doi: 10.1208/s12248-024-00979-7. AAPS J. 2024. PMID: 39467882
-
A Meta-Analysis Methodology in Stan to Estimate Population Pharmacokinetic Parameters from Multiple Aggregate Concentration-Time Datasets: Application to Gevokizumab mPBPK Model.Pharmaceutics. 2024 Aug 27;16(9):1129. doi: 10.3390/pharmaceutics16091129. Pharmaceutics. 2024. PMID: 39339167 Free PMC article.
-
Distribution Clearance: Significance and Underlying Mechanisms.Pharm Res. 2024 Jul;41(7):1391-1400. doi: 10.1007/s11095-024-03738-7. Epub 2024 Jul 9. Pharm Res. 2024. PMID: 38981900 Free PMC article.
-
Predicting the clinical subcutaneous absorption rate constant of monoclonal antibodies using only the primary sequence: a machine learning approach.MAbs. 2024 Jan-Dec;16(1):2352887. doi: 10.1080/19420862.2024.2352887. Epub 2024 May 14. MAbs. 2024. PMID: 38745390 Free PMC article.
-
Quantification of biochemical PSA dynamics after radioligand therapy with [177Lu]Lu-PSMA-I&T using a population pharmacokinetic/pharmacodynamic model.EJNMMI Phys. 2024 Apr 24;11(1):39. doi: 10.1186/s40658-024-00642-2. EJNMMI Phys. 2024. PMID: 38656678 Free PMC article.
References
-
- Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. - PubMed
-
- Jusko WJ. Guidelines for collection and pharmacokinetic analysis of drug disposition data. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics: principles of therapeutic drug monitoring. 1. Applied Therapeutics Inc; Vancouver, WA: 1980. pp. 639–680.
-
- Chiou WL. Potential pitfalls in the conventional pharmacokinetic studies: effects of the initial mixing of drug in blood and the pulmonary first-pass elimination. J Pharmacokinet Biopharm. 1979;7:527–536. - PubMed
-
- Riegelman S, Loo JC, Rowland M. Shortcomings in pharmacokinetic analysis by conceiving the body to exhibit properties of a single compartment. J Pharm Sci. 1968;57:117–123. - PubMed
-
- Vaughan DP, Hope I. Applications of a recirculatory stochastic pharmacokinetic model: limitations of compartmental models. J Pharmacokinet Biopharm. 1979;7:207–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

