Stem Cell Implants for Cancer Therapy: TRAIL-Expressing Mesenchymal Stem Cells Target Cancer Cells In Situ
- PMID: 23091539
- PMCID: PMC3468780
- DOI: 10.4048/jbc.2012.15.3.273
Stem Cell Implants for Cancer Therapy: TRAIL-Expressing Mesenchymal Stem Cells Target Cancer Cells In Situ
Abstract
Purpose: Tumor-specific delivery of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), an apoptosis-inducing peptide, at effective doses remains challenging. Herein we demonstrate the utility of a scaffold-based delivery system for sustained therapeutic cell release that capitalizes on the tumor-homing properties of mesenchymal stem cells (MSCs) and their ability to express genetically-introduced therapeutic genes.
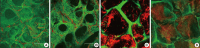
Methods: Implants were formed from porous, biocompatible silk scaffolds seeded with full length TRAIL-expressing MSCs (FLT-MSCs). under a doxycycline inducible promoter. In vitro studies with FLT-MSCs demonstrated TRAIL expression and antitumor effects on breast cancer cells. Next, FLT-MSCs were administered to mice using three administration routes (mammary fat pad co-injections, tail vein injections, and subcutaneous implantation on scaffolds).
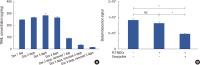
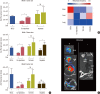
Results: In vitro cell-specific bioluminescent imaging measured tumor cell specific growth in the presence of stromal cells and demonstrated FLT-MSC inhibition of breast cancer growth. FLT-MSC implants successfully decreased bone and lung metastasis, whereas liver metastasis decreased only with tail vein and co-injection administration routes. Average tumor burden was decreased when doxycycline was used to induce TRAIL expression for co-injection and scaffold groups, as compared to controls with no induced TRAIL expression.
Conclusion: This implant-based therapeutic delivery system is an effective and completely novel method of anticancer therapy and holds great potential for clinical applications.
Keywords: Breast neoplasms; Mesenchymal stem cells; TNF-related apoptosis-inducing ligand; Tissue engineering; Tissue therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Mesenchymal stromal cell delivery of full-length tumor necrosis factor-related apoptosis-inducing ligand is superior to soluble type for cancer therapy.Cytotherapy. 2015 Jul;17(7):885-96. doi: 10.1016/j.jcyt.2015.03.603. Epub 2015 Apr 14. Cytotherapy. 2015. PMID: 25888191 Free PMC article.
-
Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells.Neurosurgery. 2009 Sep;65(3):610-24; discussion 624. doi: 10.1227/01.NEU.0000350227.61132.A7. Neurosurgery. 2009. PMID: 19687708
-
TRAIL-expressing gingival-derived mesenchymal stem cells inhibit tumorigenesis of tongue squamous cell carcinoma.J Dent Res. 2015 Jan;94(1):219-28. doi: 10.1177/0022034514557815. Epub 2014 Nov 12. J Dent Res. 2015. PMID: 25391621
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
Potential of Mesenchymal Stem Cell based application in Cancer.Int J Hematol Oncol Stem Cell Res. 2015 Apr 1;9(2):95-103. Int J Hematol Oncol Stem Cell Res. 2015. PMID: 25922650 Free PMC article. Review.
Cited by
-
TRAIL on trial: preclinical advances in cancer therapy.Trends Mol Med. 2013 Nov;19(11):685-94. doi: 10.1016/j.molmed.2013.08.007. Epub 2013 Sep 26. Trends Mol Med. 2013. PMID: 24076237 Free PMC article. Review.
-
Adenovirus platform enhances transduction efficiency of human mesenchymal stem cells: An opportunity for cellular carriers of targeted TRAIL-based TR3 biologics in ovarian cancer.PLoS One. 2017 Dec 21;12(12):e0190125. doi: 10.1371/journal.pone.0190125. eCollection 2017. PLoS One. 2017. PMID: 29267342 Free PMC article.
-
Human mesenchymal stem cells as delivery of osteoprotegerin gene: homing and therapeutic effect for osteosarcoma.Drug Des Devel Ther. 2015 Feb 17;9:969-76. doi: 10.2147/DDDT.S77116. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25733814 Free PMC article.
-
Immunotherapeutic organoids: a new approach to cancer treatment.Biomatter. 2013 Jan-Mar;3(1):e23897. doi: 10.4161/biom.23897. Epub 2013 Jan 1. Biomatter. 2013. PMID: 23507921 Free PMC article. Review.
-
HGF Gene Modification in Mesenchymal Stem Cells Reduces Radiation-Induced Intestinal Injury by Modulating Immunity.PLoS One. 2015 May 1;10(5):e0124420. doi: 10.1371/journal.pone.0124420. eCollection 2015. PLoS One. 2015. PMID: 25933295 Free PMC article.
References
-
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

